A kind of triphenylamine derivative and its preparation method and application
A derivative, triphenylamine technology, applied in the field of triphenylamine derivatives and its preparation and application, can solve the problems of limited material application, and achieve the effects of good cycle, high contrast, and convenient device preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
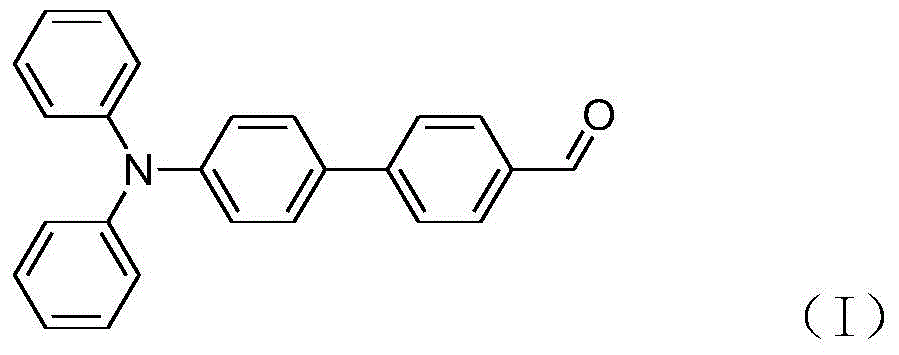
[0041] Dissolve 0.92g (5mmol) of p-bromobenzaldehyde (II), 2.17g (7.5mmol) of triphenylamine 4-boronate, and 0.1122g (0.5mmol) of palladium acetate in 10mL of deionized water / 20mL of isopropanol, and add phosphoric acid Tripotassium 1g (4.7mmol). In an air environment, react at room temperature for 11 minutes. After the reaction was quenched with saturated brine, it was extracted three times with ethyl acetate, the organic phases were combined, washed with saturated brine, and finally dried over anhydrous magnesium sulfate. Filtration, the filtrate was concentrated under reduced pressure, and the residue was separated by silica gel column chromatography, eluted with a mixed solvent with a volume ratio of petroleum ether / ethyl acetate of 30:1, and the eluent containing the product was collected, and the eluent was decompressed After evaporating the solvent, 1.57 g of product (I) was obtained as a yellow powder, with a yield of 90%. The structural confirmation of the substance...
Embodiment 2
[0043]Dissolve 0.92 g (5 mmol) of p-bromobenzaldehyde (II), 1.156 g (4 mmol) of triphenylamine 4-boronate, and 0.11 g (0.5 mmol) of palladium palladium acetate in 6.5 mL of deionized water / 13.5 mL of isopropanol, 1 g (4.7 mmol) of tripotassium phosphate was added. In an air environment, react at room temperature for 11 minutes. After the reaction was quenched with saturated brine, it was extracted three times with ethyl acetate, the organic phases were combined, washed with saturated brine, and finally dried over anhydrous magnesium sulfate. Filtration, the filtrate was concentrated under reduced pressure, and the residue was separated by silica gel column chromatography, eluted with a mixed solvent with a volume ratio of petroleum ether / ethyl acetate of 30:1, and the eluent containing the product was collected, and the eluent was evaporated under reduced pressure After solvent removal, 1.4 g of yellow powder product (I) was obtained with a yield of 75%.
Embodiment 3
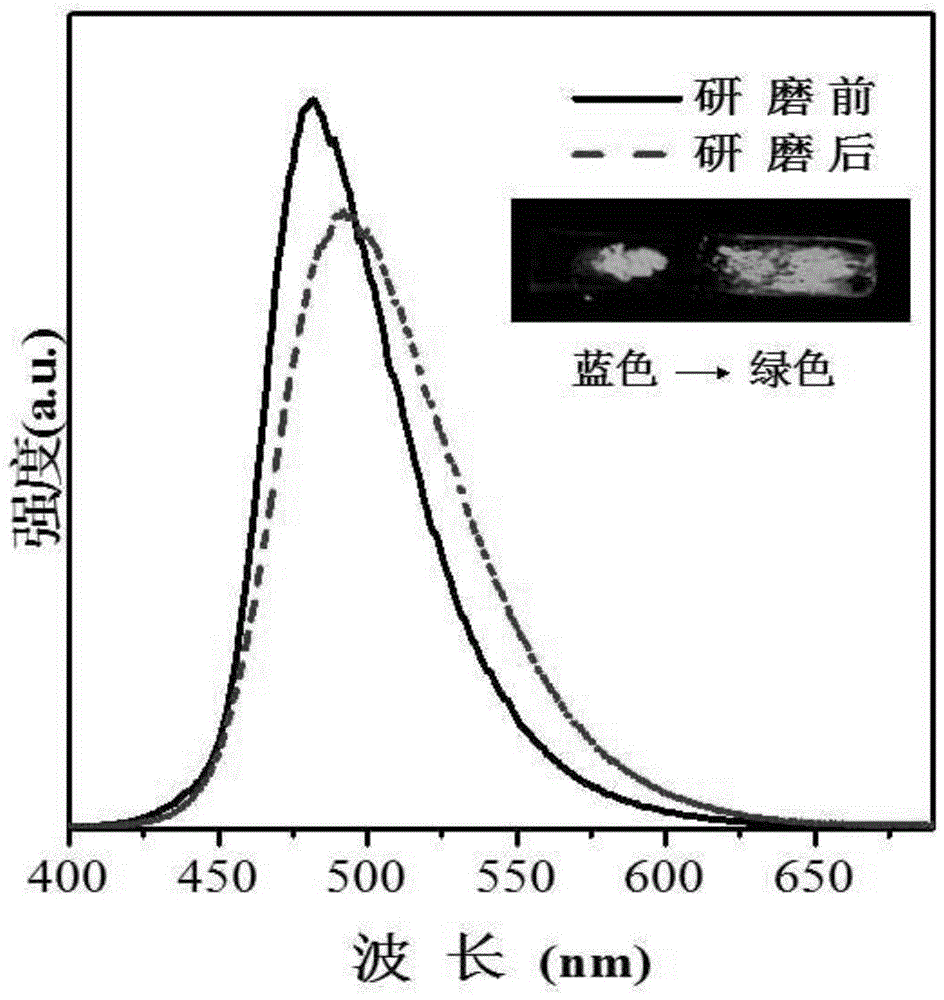
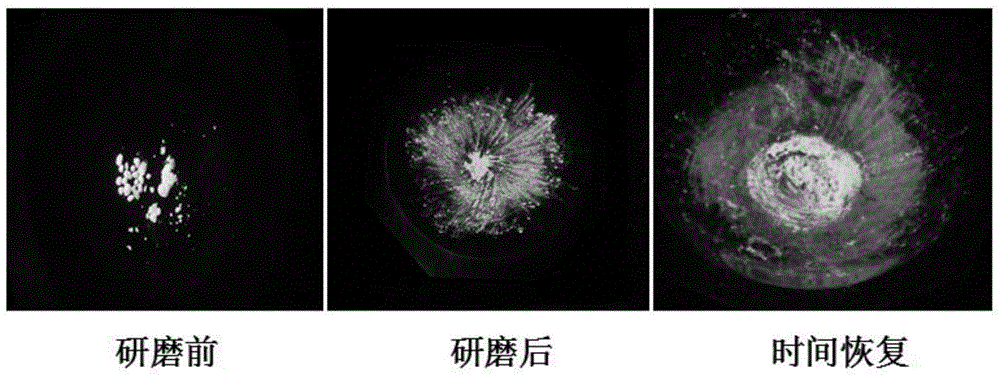
[0045] The triphenylamine derivatives solid powder (I) 0.1g that the embodiment of the present invention makes is spread on the quartz plate or is placed in the mortar, shows sky-blue fluorescence under the ultraviolet lamp, when subjected to molybdenum scraping shear force or pestle After the action, the fluorescent color under the ultraviolet lamp changes to grass green, remove the external force, and the stressed part still maintains grass green fluorescence. The photographs under UV light before and after normal pressure grinding and the solid-state fluorescence spectrum are as follows: figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com