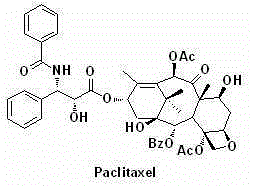
Side chain C-3 position-modified taxane analogs and preparation method thereof
A taxane and side chain technology, applied in the field of paclitaxel analogs and their synthesis, can solve the problems of low natural content, low water solubility, drug resistance, etc., and achieve the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
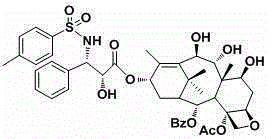
[0026] Example 1: Preparation of compound 3’-n- to prepare method of toluene-7,9,10- to remove acetyl-1-1-dehrotic hydroxybaka Pavilion VI. The structural format of this compound is:
[0027]
[0028] A.1-Demoba Kaica Pavilion VI1 (419mg, 0.6mmol) is dissolved in 20ml95%alcohol, adds 10ml water combination, and stir at room temperature for 15 hours.Use 0.2N dilute hydrochloric acid to neutralize, ethyl acetate extract, organic phase to wash three times with saturated saline, dry sodium sodium sulfate, and reduce the solvents with decompression.Cross-products with methanol and orthopedane heavy crystals, which are colorless transparent crystals 7,9,10,13-four-de-acetyl-1-oxygenbaca Pavilion VI2 is 227mg, and the yield is 87%;
[0029] B.Compound 2 (239mg, 0.5 mmol) dissolved in 18ml dichlorobial and 1.5ml methanol. After completely dissolving, add 2,2-di oxygenyl propylene (0.4ml, 2.0mmol), stir well, and add Montk10 to 24mg.Stir at room temperature for 0.5 hours; decompression d...
Embodiment 2
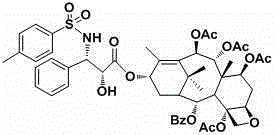
[0039] Example 2: Preparation of compounds 3’-n-benzenesulfraogl-7,9,10- to remove acetyl-1-1-dehrotic hydroxybaka Pavilion VI. The structure of the compound is:
[0040]
[0041] Methods Sedow Example 1:
[0042] 6b: 1 HNMR (500MHz ,CDCL 3 ) Δ (ppm): 3.24 (s, 1H), 3.77 (s, 3H), 4.38 (d, D, J = 2.3Hz, 1H), 4.91 (DD, J = 2.39.6Hz, 1H), 5.58 (D, J = 9.8Hz, 1H), 7.14-7.15 (m, 2H), 7.20 (T, T, J = 3.3Hz, 3H), 7.33 (T, J = 7.8Hz, 2H), 7.44 (T, J = 8.0Hz, 1H), 7.67 (D, J = 7.2Hz, 2H); 13 C-NMR (125MHz, CDCL 3 ) Δ (PPM): 22.10,27.79,52.93,59.42,67.61,68.66,744,107.87,126.83,126.27,128.64,137.35,140.54,172.39.
[0043] 7b: 1 HNMR (500MHz , CDCL 3 ) Δ (ppm): 3.24 (s, 1H), 3.77 (s, 3H), 4.38 (d, D, J = 2.3Hz, 1H), 4.91 (DD, J = 2.39.6Hz, 1H), 5.58 (D, J = 9.8Hz, 1H), 7.14-7.15 (m, 2H), 7.20 (T, T, J = 3.3Hz, 3H), 7.33 (T, J = 7.8Hz, 2H), 7.44 (T, J = 8.0Hz, 1H), 7.67 (D, J = 7.2Hz, 2H); 13 C-NMR (125MHz, CDCL 3 ) Δ (PPM): 27.03,28.84,52.64,65.17,81.74,102.55,127.44,127.65, 128.12, 1328,13...
Embodiment 3
[0047] Example 3: Preparation of compound 3’-N-Preparation method of dehuminyl-1-1-1-1-1-1-1-1-1-1-1-1-1-1-1-1-1-1-1-deodba-1-1-deodiabenyl-1.
[0048]
[0049] Methods Same Example 1:
[0050] 6C: 1 HNMR (500MHz, CDCL 3 ) Δ (ppm): 3.68 (s, 1H), 3.79 (s, 3H), 4.38 (d, D, J = 2.2Hz, 1H), 4.88 (DD, J = 2.39.8Hz, 1H), 6.36 (D, J = 9.8Hz, 1H), 7.13 (D, J = 1.2Hz, 2H), 7.19 (D, J = 3.0Hz, 1H), 7.37 (s, 1H), 7.38-7.41 (m, 3H), 7.46 (D, J = 6.8Hz, 1H); 13 CNMR (125MHz, CDCL 3
[0051] 7C: 1 HNMR (500MHz, CDCL 3 ) Δ (ppm): 1.80 (s, 3H), 1.87 (s, 3H), 3.72 (s, 3H), 4.52 (d, D, J = 5.4Hz, 1H), 5.18 (D, J = 5.4Hz, 1H), 7.13-7.17 (m, 4H), 7.19-7.22 (m, 1H), 7.27-7.34 (m, 4H); 13 CNMR (125MHz, CDCL 3 ) Δ (PPM): 27.20,28.59,52.70,65.11,81.69,100.64,127.26,127.81,128.23,128.95,137.22,139.84,170.26;
[0052] 8C: 1 HNMR (500MHz, CDCL 3 ) Δ (ppm): 1.24 (s, 3H), 1.27 (s, 3H), 1.54 (s, 6H), 1.69 (s, 3H), 1.81 (s, 3H), 1.88 (s, 6H), 1.94 (S, 3H), 2.00 (D, J = 8.8Hz, 1H), 2.06 (D, J= 11.2Hz, 1H), 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com