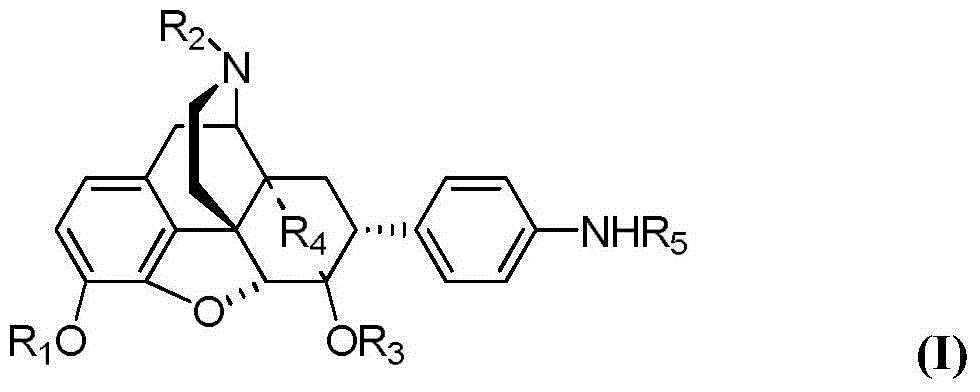
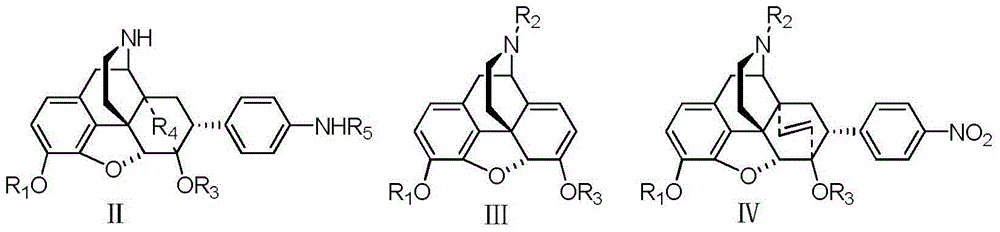
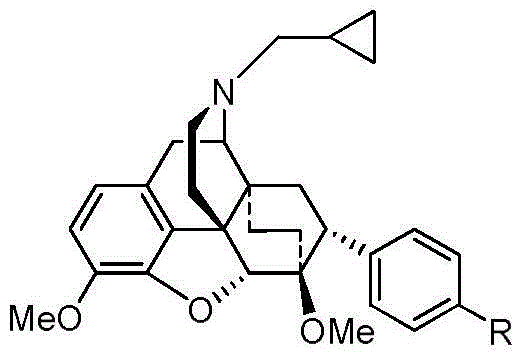
Fused ring morphines derivative, and preparation method therefor and application thereof
A derivative, cycloalkyl technology, applied in the field of fused-ring morphine derivatives, can solve the problems of restlessness, restricted development, dose-dependent hallucinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 same method can prepare IV formula compound
[0029] Preparation of 7α-(4'-nitro-phenyl)-6α,14α-endo-vinyl-tetrahydrothebaine
[0030] Add 2g (6.42mmol) of thebaine, 2.5g (0.0168mol) of p-nitrostyrene and 10mL of xylene into a 25mL three-necked flask equipped with magnetic stirring, reflux condenser and thermometer. The reaction was refluxed for 24 hours, concentrated to dryness under reduced pressure, and subjected to silica gel column chromatography, eluting with ethyl acetate / petroleum ether. 2.12 g of 7α-p-nitrophenylthebaine was obtained as light yellow foam, m.p.99-102°C, yield 71.7%. The free base was dissolved in absolute ethanol, and HCl / ether solution was added dropwise to adjust the pH to 4-5. After filtration and drying, the hydrochloride was obtained as white powder, m.p.216-218°C.
[0031] IR(KBr)υ3441,2928,1518,1346,1108,796,696cm -1 . 1 HNMR (CDCl 3 )δ8.09(dd,2H,J 1 =2.35Hz,J 2 =8.61Hz), 7.36(dd,2H,J 1 =2.35Hz,J 2 =11.35Hz),6.66(d,1...
Embodiment 2
[0032] R in the I formula can be prepared with the same method in embodiment 2 2 =C 1 -C 6 Alkyl, C 2 -C 6 Alkenyl, C 2 -C 6 Alkynyl, C 3 -C 6 Cycloalkyl, C 5 -C 7 Cycloalkenyl, C 3 -C 6 Cycloalkyl C 1 -C 6 Alkyl, C 5 -C 7 Cycloalkenyl C 1 -C 6 Alkyl, aryl, heterocyclic aryl, aryl C 1 -C 6 Alkyl, or heterocyclic aryl C 1 -C 6 Alkyl compounds and their salts
[0033] Preparation of N-cyclopropylmethyl-7α-p-nitrophenyl-6α,14α-endo-vinyl-tetrahydrothebaine hydrochloride
[0034] Add 3.4g (12.6mmol) of 7α-p-nitrophenyl-6α,14α-endo-vinyl-tetrahydrothebaine and 90ml of acetonitrile into a 250mL single-necked flask under nitrogen protection, and then add diisopropyl azodicarboxylate 7.88ml (37.8mmol), reflux reaction for 4h, stop the reaction, spin the reaction solution to dryness, add 1N HCl90ml, reflux for 4h, after the reaction is over, a large amount of solid precipitates, filter, wash the filter cake with ethyl acetate, and dry to obtain a light yellow soli...
Embodiment 3
[0036] Embodiment 3 can prepare R in I formula with method 4 is-CH 2 -CH 2 -, R 5 = Derivatives of H
[0037] Preparation of N-cyclopropylmethyl-7α-p-aminophenyl-4α,14α-endo-ethylene-tetrahydrothebaine
[0038] Add 2.63g (5.25mmol) of the compound N-cyclopropylmethyl-7α-p-nitrophenyl-4α,14α-endo-vinyl-tetrahydrothebaine, 60ml of ethanol and 5% palladium carbon in a pressure bottle 0.67g (0.315mmol), feed hydrogen, 65psi, heat at 50oC for 48h, stop the reaction, filter the reaction solution to remove palladium carbon, spin the filtrate to obtain 2.1g of white foamy solid, yield: 84.6%, melting point: 94-95oC . [MS][M+H] + :473.3.IRυ700.60,731.20,807.81,902.82,881.35,957.90,939.68,1008.38,1055.10,1092.31,1162.70,1204.44,1262.50,1328.78,1440.09,1515.67,1500.13,1623.06,2834.01,2929.68,3048.58,3360.32,3457.49cm -1 . 1 HNMR (600MHz, CDCl 3 )δ7.12(d, J=8.3Hz, 2H), 6.72(d, J=8.1Hz, 1H), 6.65(d, J=8.3Hz, 2H), 6.58(d, J=8.1Hz, 1H) ,5.29(s,2H),4.60(s,1H),3.87(s,3H),3.21(t,J=12....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com