7 alpha-substituted phenyl-6 alpha, alpha-endo-ethylidene(ethenylidene)-tetrahydrochysene paramorphine derivant or its salt, preparation method and application thereof
A technology of tetrahydrothebaine and derivatives, applied in the field of pharmacy, can solve problems such as failure to achieve success and limited bioavailability, and achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 prepares R in I formula with method 5 = H, NO 2 Compounds and their salts
[0036] Preparation of 7α-(4'-nitro-phenyl)-6α,14α-endo-vinylidene-tetrahydrothebaine and its hydrochloride
[0037] Add 2g (6.42mmol) of thebaine, 2.5g (0.0168mol 6) of m-nitrostyrene and 10mL of xylene into a 25mL three-necked flask equipped with magnetic stirring, reflux condenser and thermometer. The reaction was refluxed for 24 hours, concentrated to dryness under reduced pressure, and subjected to silica gel column chromatography, eluting with ethyl acetate / petroleum ether. 2.12 g of 7α-p-nitrophenylthebaine was obtained as light yellow foam, m.p.99-102°C, yield 71.7%. The free base was dissolved in absolute ethanol, and HCl / ether solution was added dropwise to adjust the pH to 4-5. After filtration and drying, the hydrochloride was obtained as white powder, m.p.216-218°C.
[0038] IR(KBr)υ3441, 2928, 1518, 1346, 1108, 796, 696cm -1 . 1 H NMR (CDCl 3 )δ8.09 (dd, 2H, J 1...
Embodiment 2
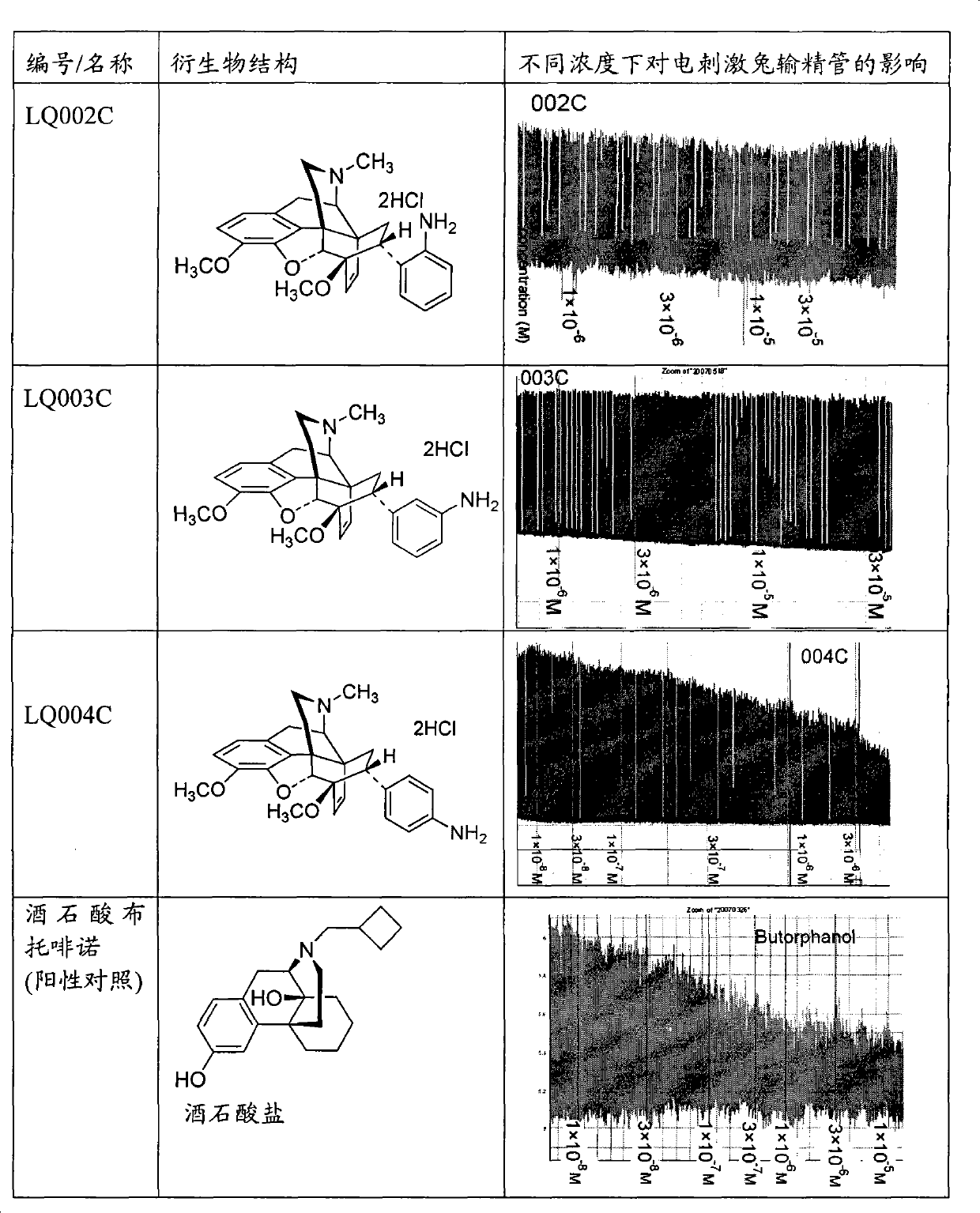
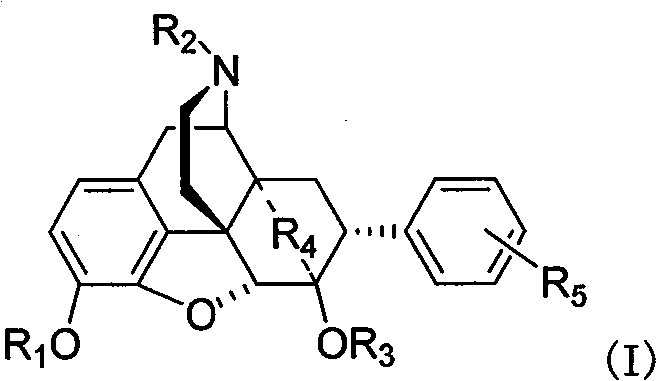
[0039] Embodiment 2 prepares R in I formula with method 1 =R 2 =R 3 =CH 3 , R 4 =-CH=CH-,R 5 =o-NH 2 Compound hydrochloride (LQ002C) and R 1 =R 2 =R 3 =CH 3 , R 4 =-CH=CH-,R 5 = m-NH 2 Compound Hydrochloride (LQ003C)
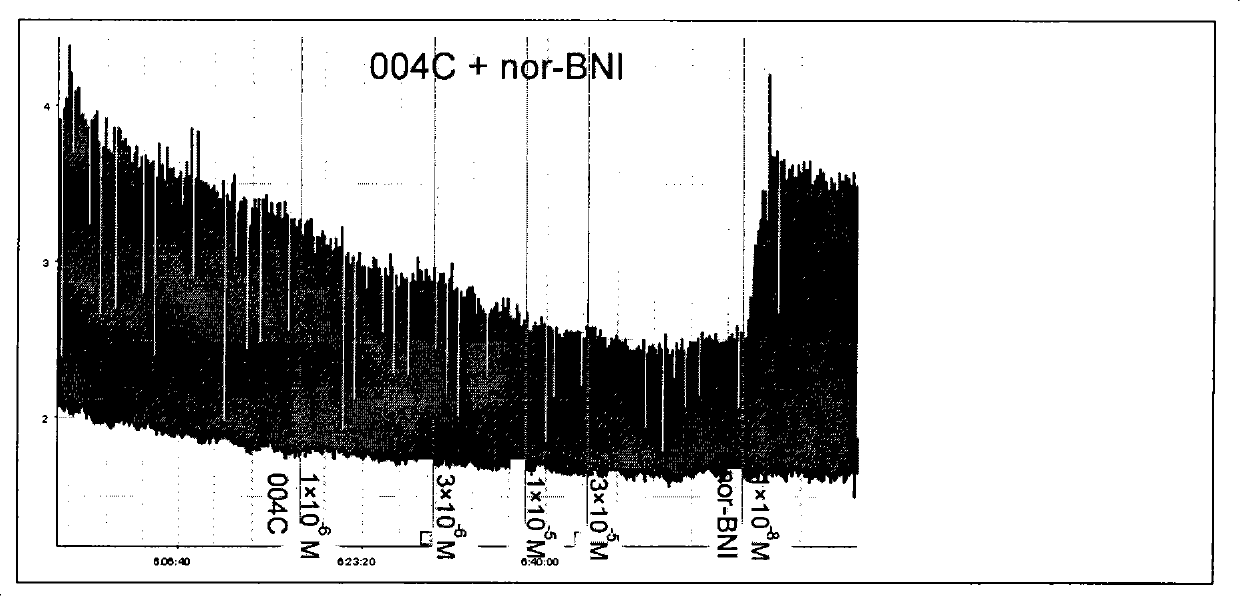
[0040] Preparation of 7α-(4'-amino-phenyl)-6α,14α-endo-vinylidene-tetrahydrothebaine and its hydrochloride (LQ004C)
[0041] Add 1g (2.17mmol) 7α-(4'-nitro-phenyl)-6α,14α-endo-ethenylidene (en)yl- Tetrahydrothebaine, 60mL absolute ethanol, keep warm at 60°C. Add 1.5 mL (25.9 mmol) of 85% hydrazine hydrate dropwise, add 1 part of Raney Ni (catalytic amount), filter while hot to remove excess Raney Ni, and concentrate the filtrate to dryness to obtain 1.00 g of white foam. Methanol was recrystallized to obtain 0.73 g of off-white needle crystals, yield 77.7%, m.p.200.0-202.2°C. After dissolving the free base in absolute ethanol, add HCl / ether solution dropwise to adjust the pH to 4-5, filter and vacuum-dry to obtain its hydrochloride, white powder,...
Embodiment 3
[0042] Example 3 Preparation of 7α-(2'-guanidino-phenyl)-6α, 14α-endo-ethenide(en)yl-tetrahydrothebaine bistrifluoroacetate and 7α-(3'- Guanidino-phenyl)-6α,14α-endo-ethenylidene-tetrahydrothebaine trifluoroacetate
[0043] Preparation of 7α-(2'-guanidino-phenyl)-6α,14α-endo-vinylidene-tetrahydrothebaine bis-trifluoroacetate
[0044] Add 100mg (0.232mmol) of 7α-(4'-amino-phenyl)-6α,14α-endo-ethhenylidene-tetrahydropyrrole to a 25mL three-necked flask with magnetic stirring, air condenser and addition funnel Baine, 90mg (0.31mmol) Boc-protected methylthiourea, 50mg (0.184mmol) HgCl 2 , 5 mL DMF and 80 uL triethylamine. Reaction 2d at room temperature. Add 10% (g / g) Na to the filtrate 2 CO 3 Mix 30mL of the solution and 50mL of ethyl acetate, shake well, let stand for liquid separation, and discard the aqueous phase. Wash the organic phase with distilled water, add a sufficient amount of anhydrous Na after the organic phase is combined 2 SO 4 dry. Filter and concentrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com