Nanoemulsion eyedrop composition containing cyclosporine and method for preparing same
A technology of cyclosporine and composition, applied in the field of nanoemulsion eye drop composition containing cyclosporine and its preparation, can solve the problems of large manufacturing equipment, difficulty in ensuring consistent quality of preparation batches, and acceleration of emulsion separation. layer process etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment Embodiment 1
[0068] Experimental example 1. prepare nanoemulsion according to the type and content variation of non-aqueous solvent and measure its average particle size
[0069] Nanoemulsion compositions were prepared with different amounts of castor oil, labrafac lipophilic WL1349 or miglyol812, and then their average particle size was measured. The specific method for preparing the nanoemulsion composition is as follows. At 600rpm to 800rpm and 70°C, by using a stirrer (Super-Nuova TM Multi-place, ThermoScientific) Cyclosporine A was mixed with non-aqueous solvent in the amount shown in Table 1 below and completely dissolved. An oil phase was prepared by adding the hydrophilic and hydrophobic emulsifiers in the amounts shown in Table 1 to the mixed solution prepared above and mixing it thoroughly by stirring it. The prepared solution was cooled at room temperature, and the oil phase was placed into an aqueous solvent to wash the oil phase several times, then at 400-500rpm and at room ...
experiment Embodiment 2
[0072] Experimental Example 2. Preparation of nanoemulsions and measurement of their average particle size according to the type and varying amount of emulsifiers
Embodiment 1
[0073] The nanoemulsion compositions of Examples 16-36 were prepared by the same method described in Experimental Example 1 by using castor oil as the non-aqueous solvent, and varying the amount of castor oil and the type and amount of emulsifier. The compositions and amounts of the prepared nanoemulsion compositions are shown in Table 2 below.
[0074]
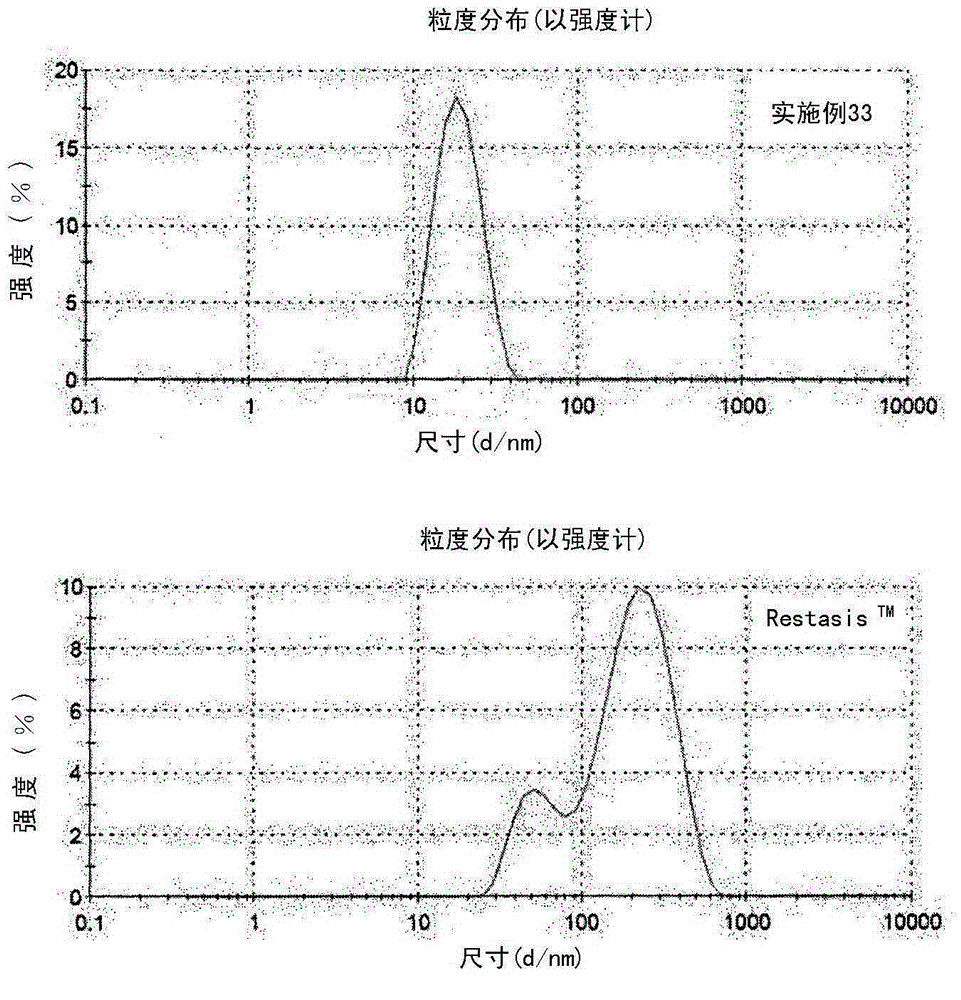
[0075] As shown in Table 2, it was confirmed that in Examples 16-34, the average particle size of the nanoemulsion composition was 100 nm or less, and its particle size distribution was very narrow. Therefore, it was confirmed that when cyclosporine, castor oil, hydrophilic emulsifier, hydrophobic emulsifier, and aqueous solvent were mixed in the above composition, an ophthalmic nanoemulsion having an average particle size of 100 nm or less and a narrow particle size distribution could be prepared combination.
[0076] In Table 2, Example 35 and Example 36 are compositions prepared by using only hydrophilic emulsifiers with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com