Application of monoclonal antibody in treating psoriasis
A monoclonal antibody and psoriasis technology, applied in the direction of antibodies, medical preparations containing active ingredients, skin diseases, etc., can solve the problems of large side effects and easy recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
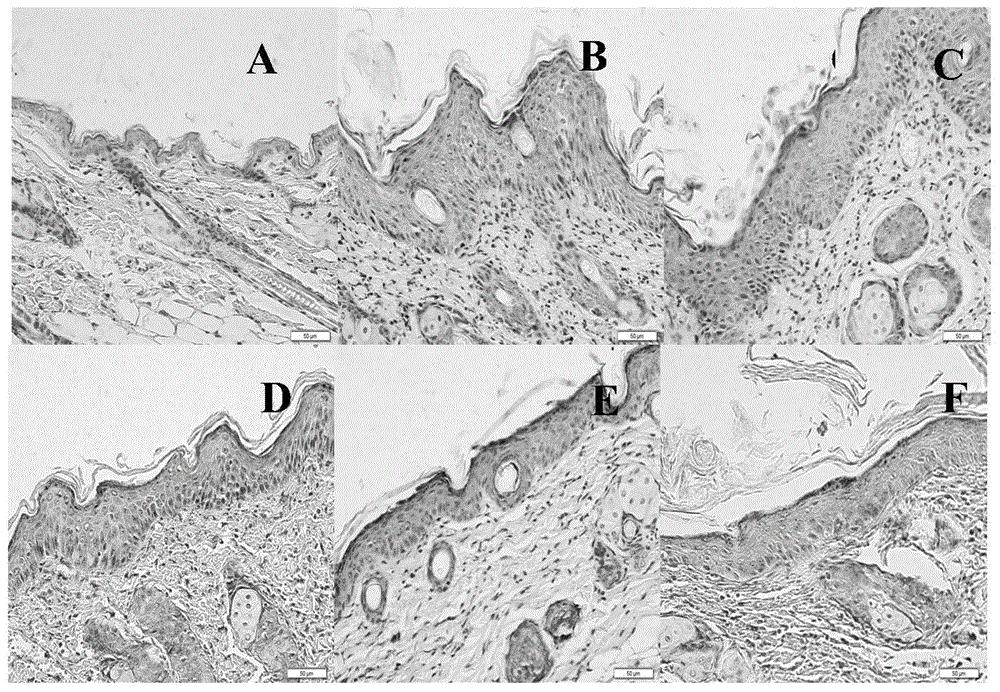
[0046] Embodiment 1 Psoriasis therapeutic effect of bifunctional monoclonal antibody of the present invention
[0047] 1. Method
[0048] 1.1 Experimental objects and materials:
[0049] Balb / c mice, male, weighing 18-22 grams, Beijing Huafukang Biotechnology Co., Ltd., the quality certificate of experimental animals: SCXK (Beijing) 2009-0007.
[0050] Imiquimod ointment: Sichuan Mingxin Pharmaceutical Co., Ltd., content: 5%, batch number: 130901;
[0051] IBI-B: Innovent Biopharmaceutical (Suzhou) Co., Ltd., concentration: 40mg / ml, batch number: E20140402;
[0052] Methotrexate: Jiangsu Hengrui Pharmaceutical Co., Ltd., specification: 100mg / bottle, batch number: 20131103
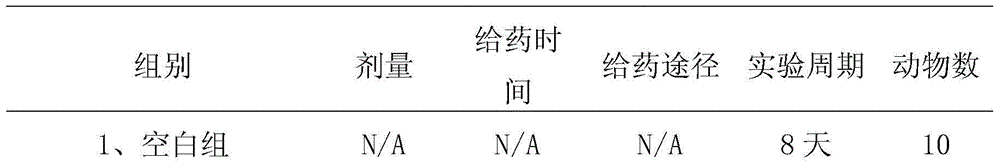
[0053] 1.2 Grouping and administration
[0054] The mouse model of psoriasis was prepared by referring to the method of Leslie et al. [1]. According to the previous research results of our laboratory, Balb / c male mice were anesthetized by intraperitoneal injection of pentobarbital sodium (80mg / kg), and th...
Embodiment 2
[0124] Example 2 The inhibitory effect of the bifunctional monoclonal antibody of the present invention on the VEGF signaling pathway
[0125] 1. Experimental materials:
[0126] EndoGRO-VEGFCompleteCultureMediaKit: MilliporeSCME002
[0127] DMEM medium: Gibco11966-025
[0128] VEGF 165 : Peprotech100-20
[0129] CCK-8: Sigma96992
[0130] 2. Experimental steps:
[0131] 2.1 Digest and count HUVECs with good growth status.
[0132] 2.2 Resuspend endothelial cells with Millpore medium to 2X10 4 cells / ml, spread 2000 / well HUVECs (100ul) on a 96-well plate.
[0133] 2. After 324 hours, the medium was aspirated and replaced with DMEM dual medium containing 10ng / ml VEGF and 35nM IgG, VEGF-Trap, CR1, IBI-B, and a negative control without VEGF and a positive control containing VEGF but no antibody were set. A blank control with only PBS was also included. Three replicate wells were set up in each group for parallel experiments.
[0134] 2.4 After another 48 hours, add 10ul ...
Embodiment 3
[0138] Example 3 The inhibitory effect of the bifunctional monoclonal antibody of the present invention on the complement signaling pathway
[0139] 1.CH50 hemolysis test
[0140] 1.1 Experimental materials:
[0141] Sheep red blood cells: Guangzhou Ruite 007001
[0142] Hemolysin: Guangzhou Ruite
[0143] Human complement serum: SigmaS1764
[0144] Buffer GVB0: 0.1% gelatin, 5mM barbiturate, 145mM NaCl, adjust the pH to 7.2-7.4 and dilute to 1L
[0145] GVB++: 0.1% gelatin, 5mM barbiturate, 145mMNaCl, 0.15mM CaCL2, 0.5mMMgCL2, adjust the pH to 7.2-7.4 and then dilute to 1L
[0146] 0.1M sucrose GVB: Add 5.14g sucrose to 150ml GVB0
[0147] GVB-EDTA: 0.1% gelatin, 5mM barbiturate, 145mM NaCl, 10mM EDTA, adjust the pH to 7.2-7.4 and dilute to 1L
[0148] The above buffer nights were sterilized by high temperature and high pressure sterilizer before use
[0149] 1.2 Experimental steps:
[0150] 1.2.1 Sensitized sheep red blood cells
[0151] a. Wash sheep erythrocytes 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com