Preparation and application of hepatitis C virus recombinant protein
A virus and protein technology, applied in the direction of anti-viral immunoglobulin, application, viral peptide, etc., can solve the problem of not being broad-spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
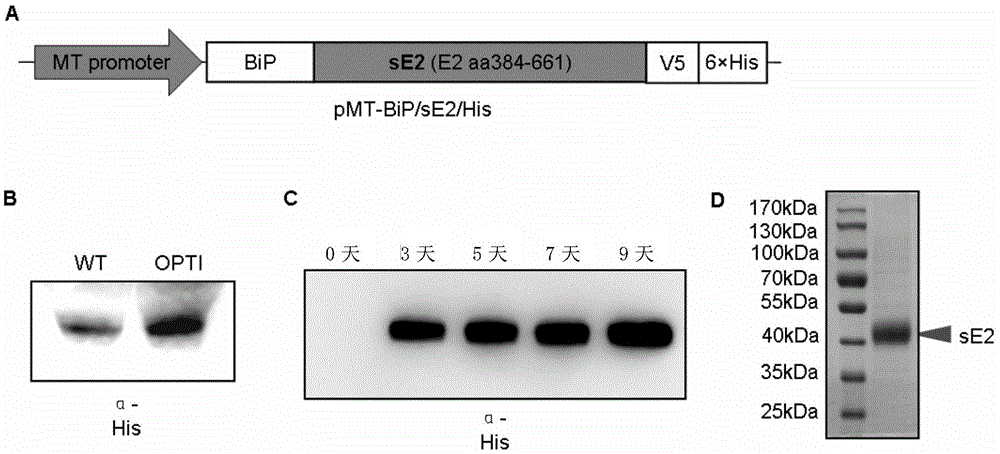
[0221] Expression of embodiment 1sE2 in fruit fly S2 cell
[0222] Recombinant plasmids used to express HCVsE2 protein in Drosophila S2 cells such as figure 1 As shown in A, the sE2 wild-type coding gene or the codon-optimized gene encodes the target protein in the extracellular region of the E2 protein (including amino acids 384-661). Drosophila S2 cells were transiently transfected with vectors expressing the wild-type sE2 coding gene or the codon-optimized gene, and 2×10 cells were removed 48 hours after transfection. 6 Cells were added chromium chloride with a final concentration of 5 μM to induce expression, and the supernatant was collected after 72 hours to detect that the optimized sE2 expression was significantly enhanced ( figure 1 B). Therefore, the inventors selected codon-optimized expression vectors for subsequent experiments. Drosophila S2 cells were co-transfected with pMT-Bip / sE2opti / His and pCoBlast, and the monoclonal cell line sE2A was obtained after bla...
Embodiment 2
[0224] The characteristic analysis of embodiment 2sE2 protein
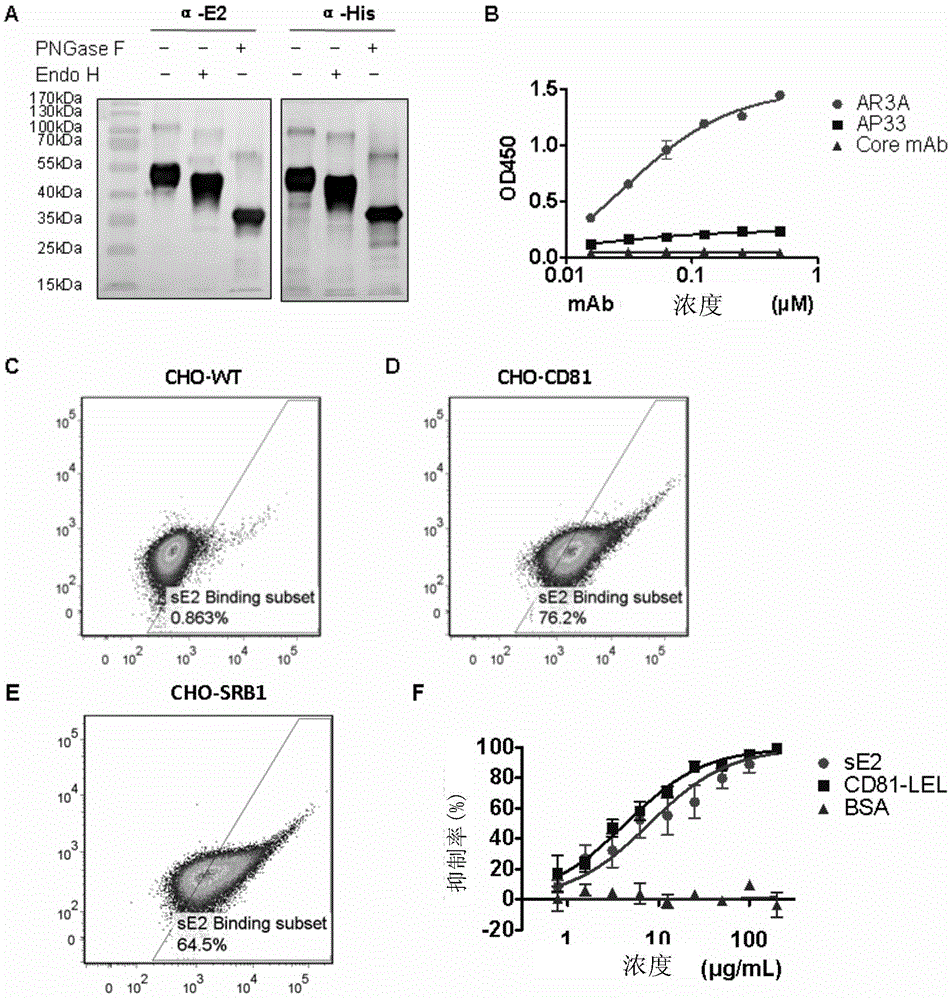
[0225] The purified sE2 protein was detected by Western blot with His monoclonal antibody and E2 monoclonal antibody (AP33) respectively, and both produced about 46kDa positive bands, indicating that the protein was indeed the target sE2 protein ( figure 2 A). After deglycosylation of sE2 protein was treated with PNGaseF, it was detected with the same antibody, and the positive band position was found to be about 34kDa ( figure 2 A), suggesting that the sE2 protein expressed in S2 insect cells has glycosylation modification.
[0226] Through ELISA detection, sE2 can well bind to the monoclonal antibody AR3A that recognizes discontinuous conformational epitopes, and its ELISA reading value is positively correlated with the monoclonal antibody concentration; although sE2 has a certain binding to the monoclonal antibody AP33 that recognizes linear epitopes, the reaction is not strong ( figure 2 B). This result ...
Embodiment 3
[0230] Embodiment 3sE2 can specifically detect HCV infection patient's serum
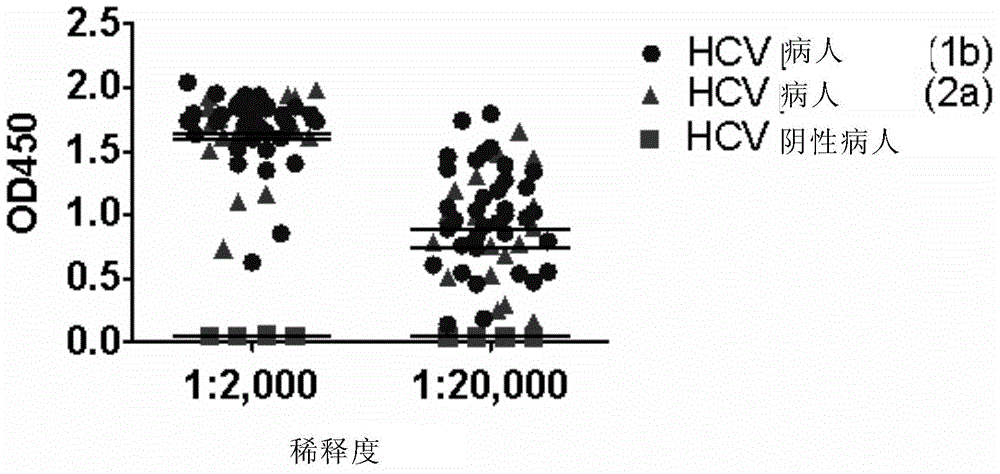
[0231] In order to determine whether sE2 can specifically bind to HCV-specific antibodies in HCV-infected persons, the inventors coated the ELISA plate with sE2 protein, and then used diluted healthy human serum and two subtypes (1b and 2a) hepatitis C patient serum respectively The incubation is followed by detection of specific IgG antibodies binding to sE2.
[0232] The results showed that compared with healthy human serum, patient serum produced a high binding activity, and the average OD value was still as high as 0.5-1.0 when diluted at 1:20000 ( image 3 ), indicating that sE2 can efficiently, specifically, and broadly bind the anti-E2 antibody in the serum of infected patients. Therefore, sE2 can be used to develop a simple and rapid diagnostic kit for HCV infection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com