Polyethylene glycol-modified human interferon and human antibacterial peptide fusion protein
A fusion protein and polyethylene glycol technology, applied in the field of bioengineering, can solve problems such as small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
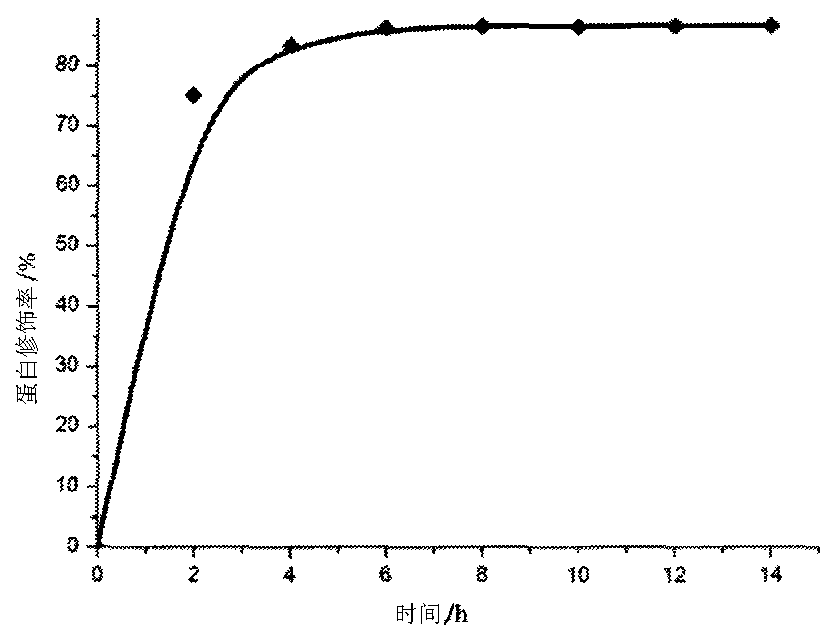
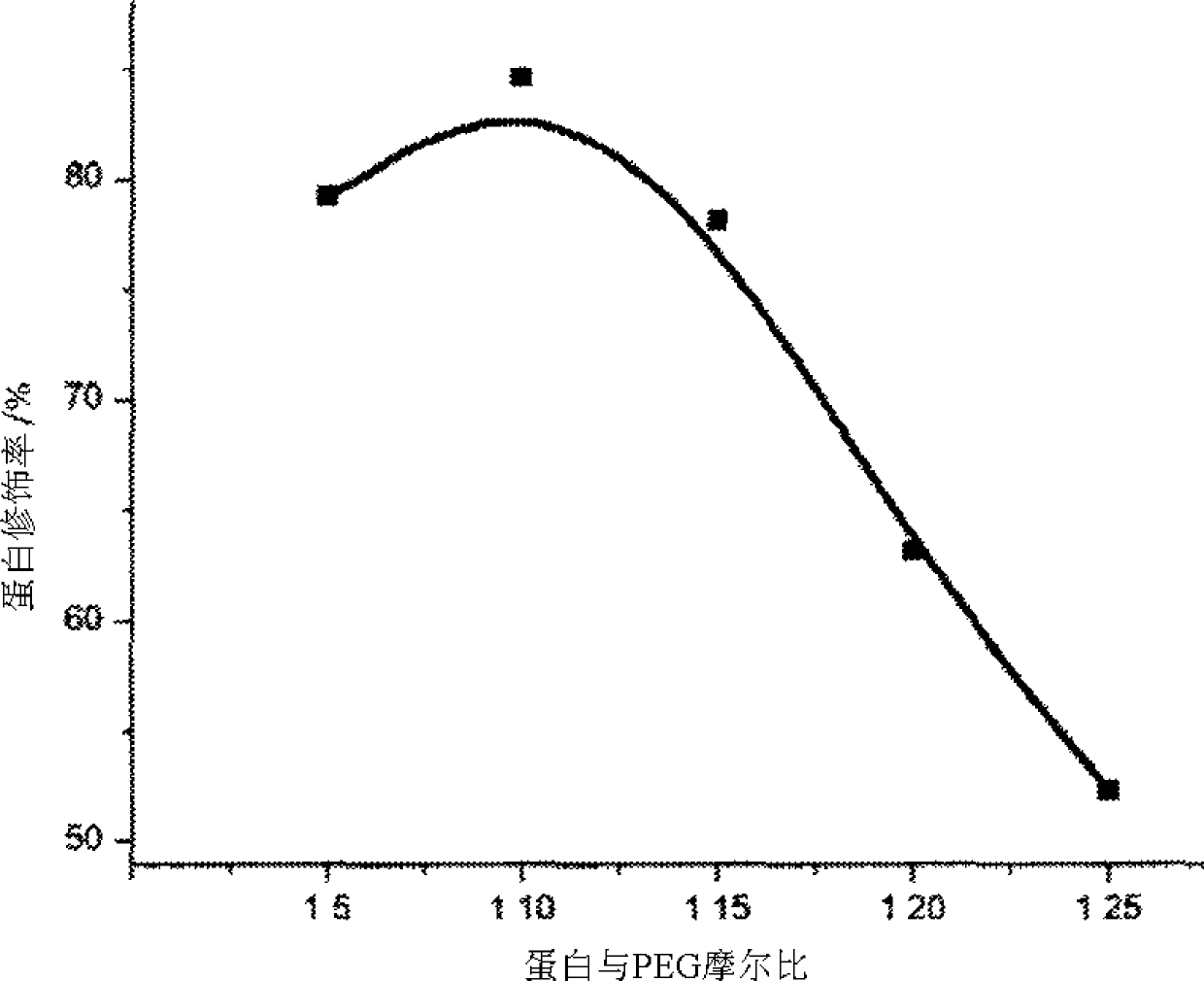
[0035] The present invention will be further described below in conjunction with the accompanying drawings and implementation examples, but the present invention is not limited thereto.
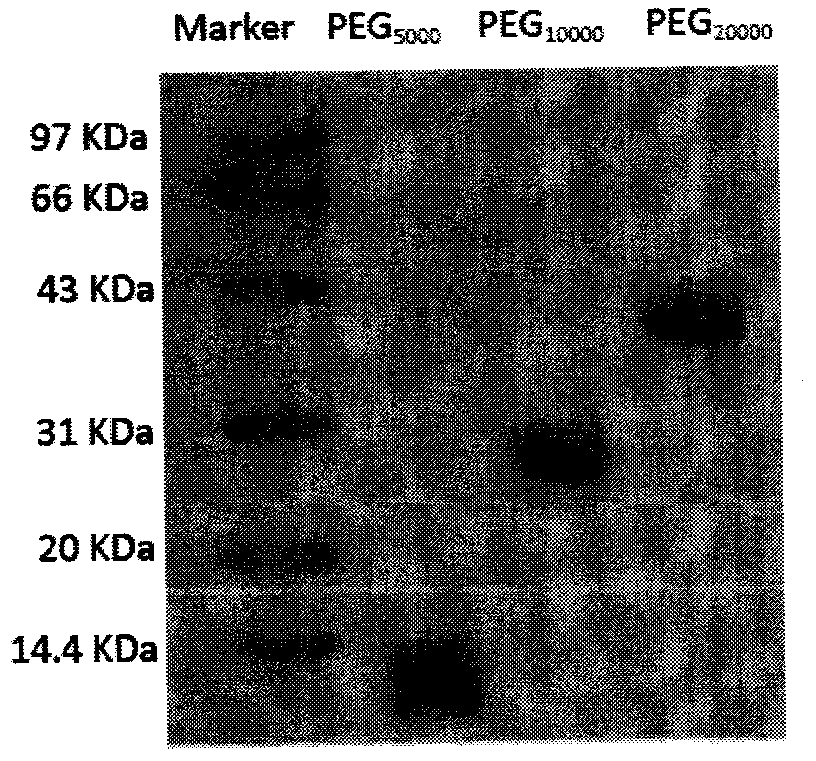
[0036] Apparent molecular weight of 1PEG
[0037] The SDS-PAGE results of PEG with three molecular weights are shown in figure 1 .
[0038] Compared with protein markers, PEG 5000 The molecular weight is about 14000, PEG 10000 The molecular weight is about 29000, PEG 20000 The weight is about 40000, which is larger than the actual molecular weight. PEG 5000 with PEG 10000 About 3 times larger, PEG 20000 About 2 times larger. The reason for the large size is related to the fact that the molecular size measured by SDS-PAGE is based on the chain length as the standard. Under the condition of the same chain length, the average molecular weight of amino acids constituting the chain length unit is larger than that of ethylene glycol, so in the system with protein as the standard control, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com