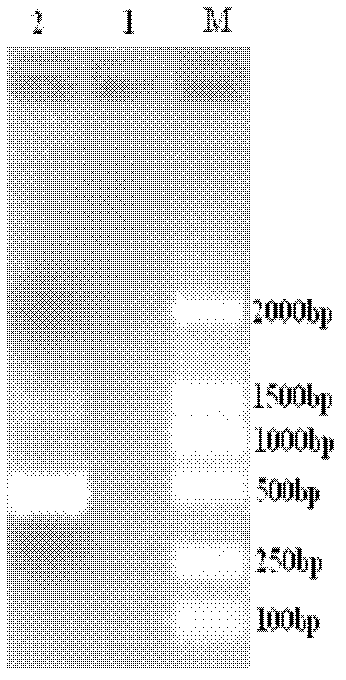Preparation method of core fragment rIFN (interferon)-alpha1of recombinant chicken IFN-alpha
A core fragment, CHA-S2 technology, applied in the field of preparation of recombinant chicken IFN-α core fragment rIFN-α1, can solve the problems of limiting chicken interferon preparation and application, unsatisfactory antiviral activity of expression products, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Primer Design Synthesis and Cloning of Target Gene
[0034] Refer to the gene sequence of chicken α-interferon that has been registered in GenBank (accession number: chicken IFN-α: GGU07868) to select external primers that can amplify chicken α-interferon, and use Primer Premier 5.0 to design and remove the signal peptide and some non- The upstream and downstream internal primers of the fragment are required (see Table 1). Among the internal primers, the upstream internal primer IFN-αCHA-S2 has an EcoR I restriction site (underlined), and the downstream internal primer IFN-αCHA-X2 has a Hind III restriction site (underlined). Extract the total RNA of chicken spleen, reverse transcribe it into cDNA, and use the cDNA as a template to perform the first round of PCR amplification with external primers. The PCR amplification steps are: 1. Pre-denaturation at 94°C for 5 minutes, 2. Denaturation at 94°C for 1 minute, 3. Anneal at 55°C for 1min 40s, 4. Extend at 72°C...
Embodiment 2
[0036] Example 2 Construction and Prokaryotic Expression of Recombinant Expression Vector (pET-30-IFN-α1)
[0037] Recover the target fragment IFN-α1 by double digestion with restriction endonucleases EcoR I and Hind III, and double digestion of the pET-30 expression vector at the same time, and then ligate the digested target fragment and vector with T4 DNA ligase overnight at 16°C , Construction of recombinant expression plasmid pET-30-IFN-α1. After transforming and culturing the recombinant plasmid, screen positive clones, extract the recombinant plasmid, and send it to Shanghai Bioengineering Co., Ltd. for sequencing verification (for the sequence of the gene fragment encoding chicken IFN-α1, see image 3 ).
[0038] The Escherichia coli BL21 with the correctly sequenced recombinant plasmid was shaken overnight in LB culture medium containing kanamycin resistance, and the overnight culture solution was added to LB culture medium containing kanamycin resistance for further...
Embodiment 3
[0039] Example 3 Purification of recombinant protein (rIFN-α1)
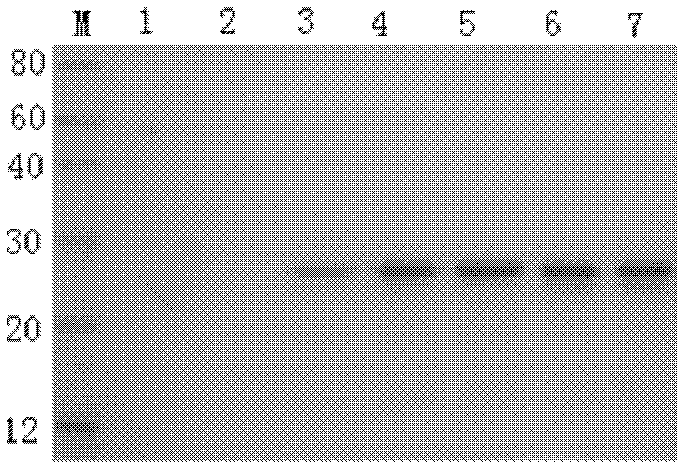
[0040] Add 1L of the bacterial solution to IPTG (final concentration: 1.0mmol.L-1) to induce expression for 4 hours, then centrifuge at 5000g for 15 minutes at 4°C, discard the supernatant, and use 1:20 (w / v) bacterial cell lysate to weigh Suspension was precipitated, sonicated, and centrifuged at 12,000 g at 4°C for 20 min; the precipitate was collected from the expressed rIFN-α1, and the inclusion body was washed sequentially with inclusion body washing solution at 1:20 (W / V). Centrifuge at 8000g for 20min, and the precipitate is inclusion body. Also follow the molecular cloning guidelines, and finally use 10mmol / L, 50mmol / L, 100mmol / L, 150mmol / L imidazole eluents to elute the target protein. The purified product was carried out on 12% SDS-PAGE to measure the purification efficiency (see figure 2 ). It has been determined that the content reaches 0.241 mg / ml, which is significantly higher than the expressio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com