Preparation and application of Al<3+> fluorescent probe based on rhodamine B derivative
A technology of fluorescent probes and derivatives, applied in the direction of fluorescence/phosphorescence, luminescent materials, chemical instruments and methods, etc., to achieve high yield, low detection limit, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
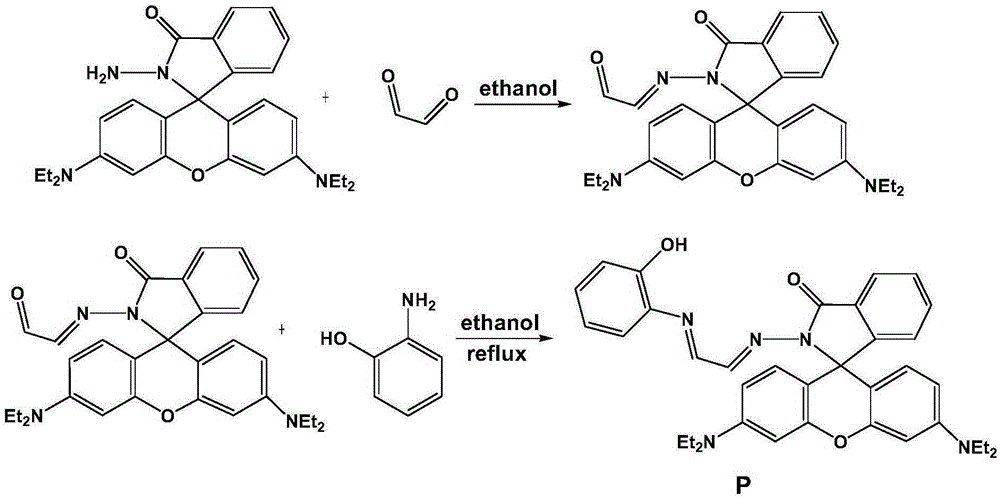
[0022] Synthesis of rhodamine B derivative P compound shown in formula 1 (see figure 1 ):
[0023] 1mmol rhodamine B hydrazinolysis product (X.F.YangX.Q.Guo, Y.B.Zhao, Talanta, 2002, 57, 883890.) and 5mmol glyoxal in absolute ethanol, stirred at room temperature for 6-8h, filtered, and then used Wash with absolute ethanol and dry to obtain the intermediate compound.
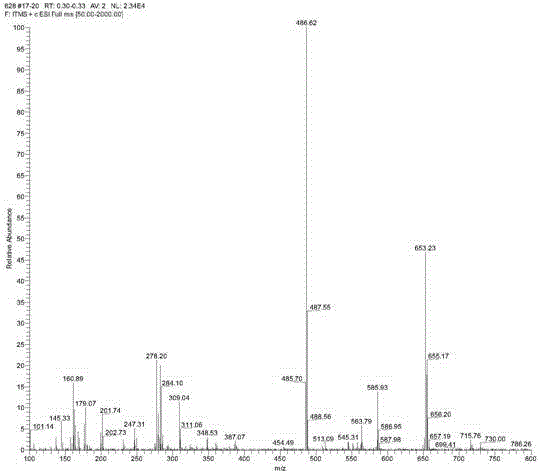
[0024] Then the obtained intermediate was reacted with o-aminophenol in absolute ethanol at 80°C for 4 hours at a molar ratio of 1:1. Petroleum ether-ethyl acetate is used as eluent to obtain yellow solid P through chromatographic column separation; Wherein, petroleum ether:ethyl acetate (v:v)=5:1, productive rate 85% (see figure 2 and 3 ).
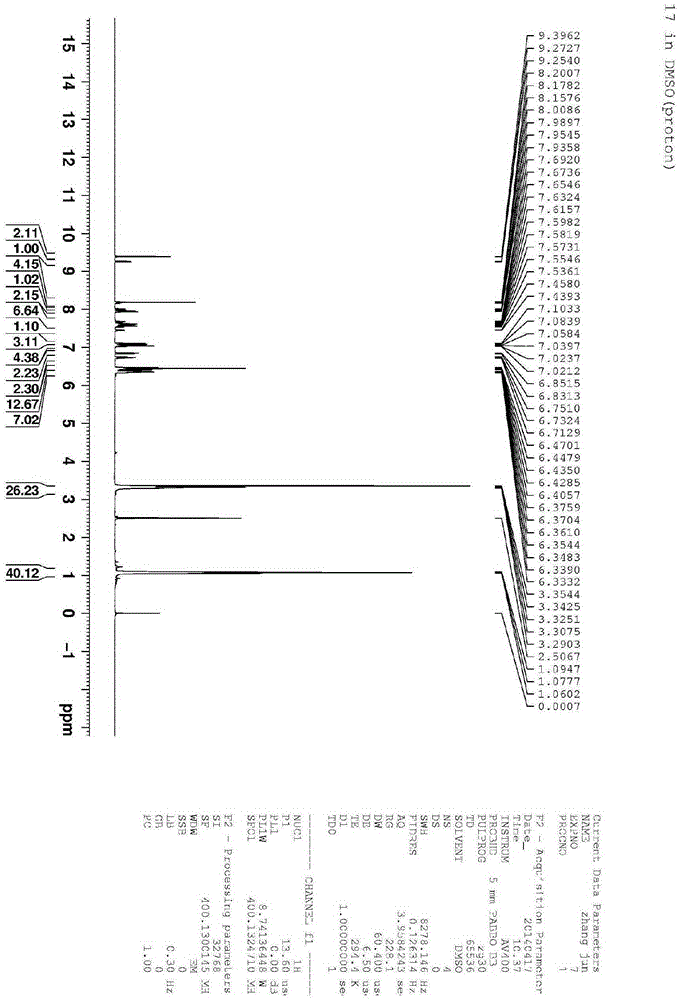
[0025] From figure 2 Molecular ion peaks and image 3 The chemical shift of H in can be used to know the formation of rhodamine B derivative P.
[0026] Rhodamine B derivatives (P) to Al 3+ optical recognition
[0027] 1) The diluent is: in ethanol / water (9 / 1, v / v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com