Method for optimized preparation of inactivated vaccine and/or attenuated live vaccine
A technology of attenuated live vaccines and inactivated vaccines, applied in the field of preparation of whole virus vaccines, can solve the problems of low immunogenicity, lack of in-depth understanding of viruses, and failure to achieve protective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 The half-infection of cells and the half-infection of chicken embryos of several viruses
[0032] 9-11-day-old SPF chicken embryos were taken to prepare chicken embryo fibroblasts (CEF), CDV, MDV and IBV were inoculated on CEF for 48 hours, harvested and the culture supernatant was repeatedly frozen and thawed 3 times, and then inoculated on CEF cells again Continue to culture, and so on to 3 generations. The virus solution was diluted 10 times and inoculated into 96-well plates or chicken embryos. CDV measurement of cell half-infectious dose (TCID) 50 ), MDV used cytopathic plaque formation to measure TCID 50 , IBV uses the half-infectious dose of chicken embryos EID 50 , to determine the virulence of three viruses. The results are shown in Tables 1-3.
[0033] Table 1 The influence of the half-infected amount of CDV cells (indirect immunofluorescence method)
[0034]
[0035] Calculated according to Reed and Muench formula: distance ratio=(lesion ra...
Embodiment 2
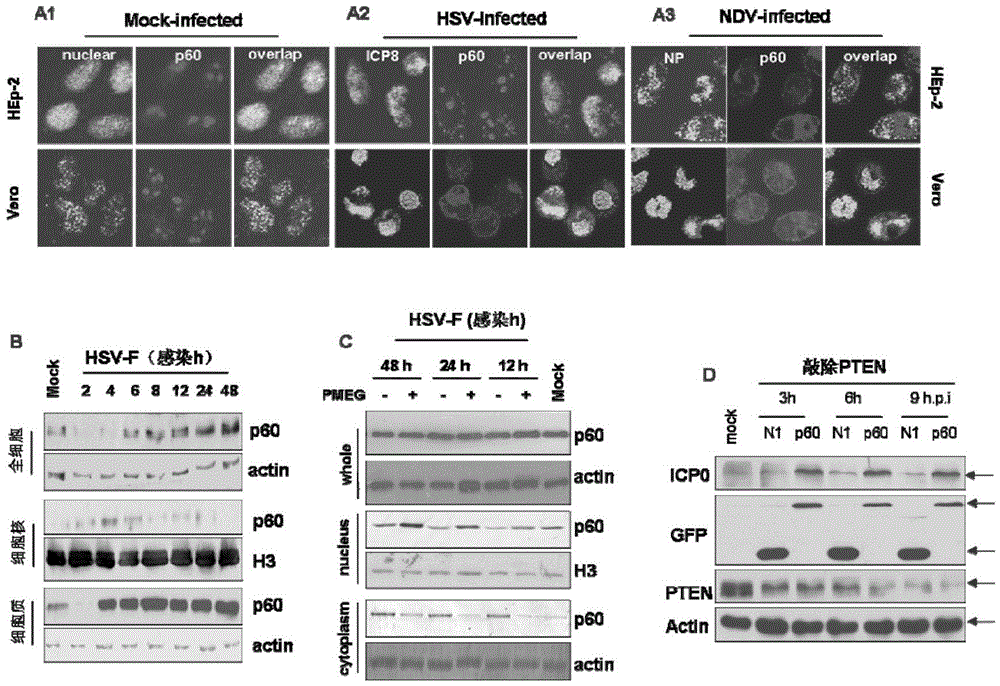
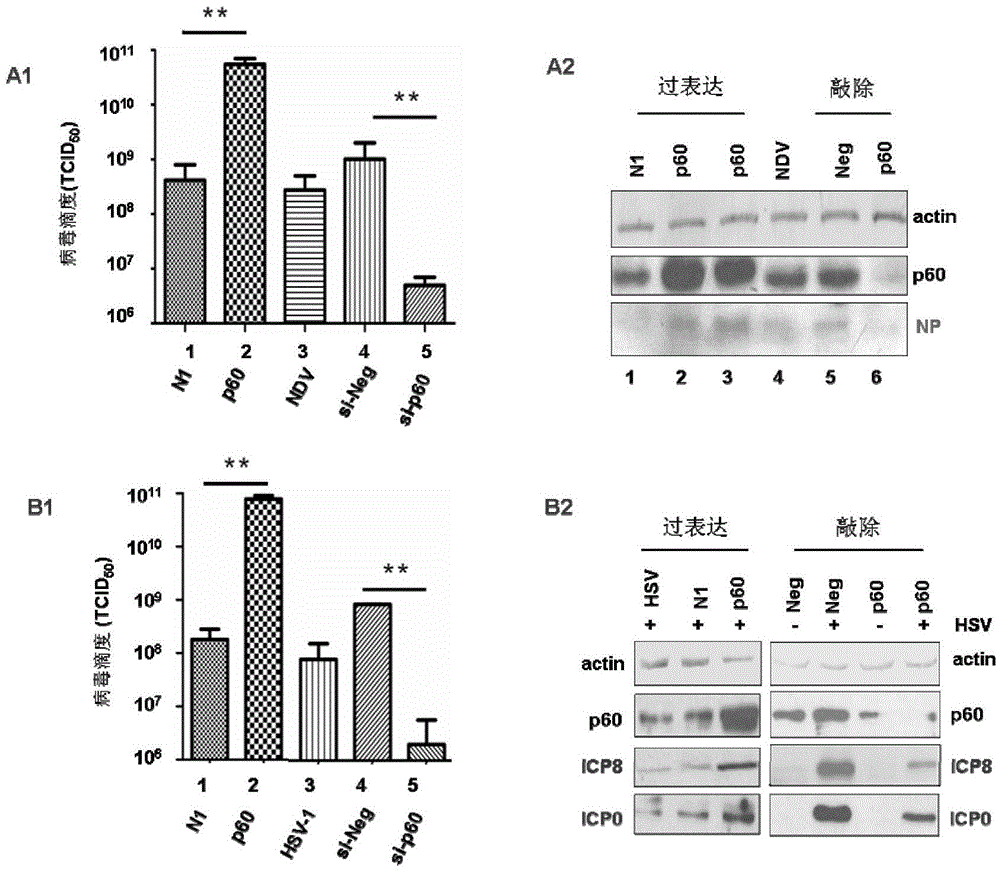
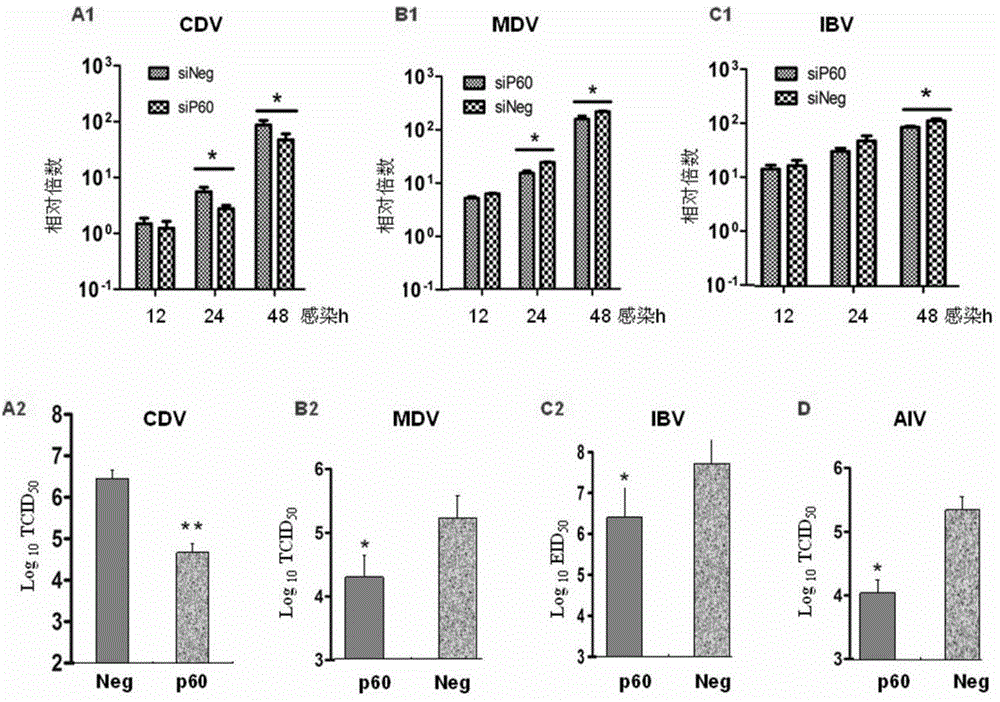
[0043] Example 2 Effect of knockout and overexpression of P60 on virus virulence (immunoblotting, lesion method and RT-PCR method)
[0044] 1. Preparation of cells
[0045] Passaging Hep-2 and Vero cells: the growth medium is DMEM medium containing 5% fetal bovine serum and 100U penicillin-streptomycin, and the maintenance medium is DMEM medium containing 2% fetal bovine serum and 100U penicillin-streptomycin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com