A method for optimizing the preparation of inactivated vaccines and/or live attenuated vaccines
A technology of attenuated live vaccines and inactivated vaccines, which is applied in the field of preparation of whole virus vaccines, and can solve problems such as lack of in-depth understanding of viruses, incompleteness, and low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
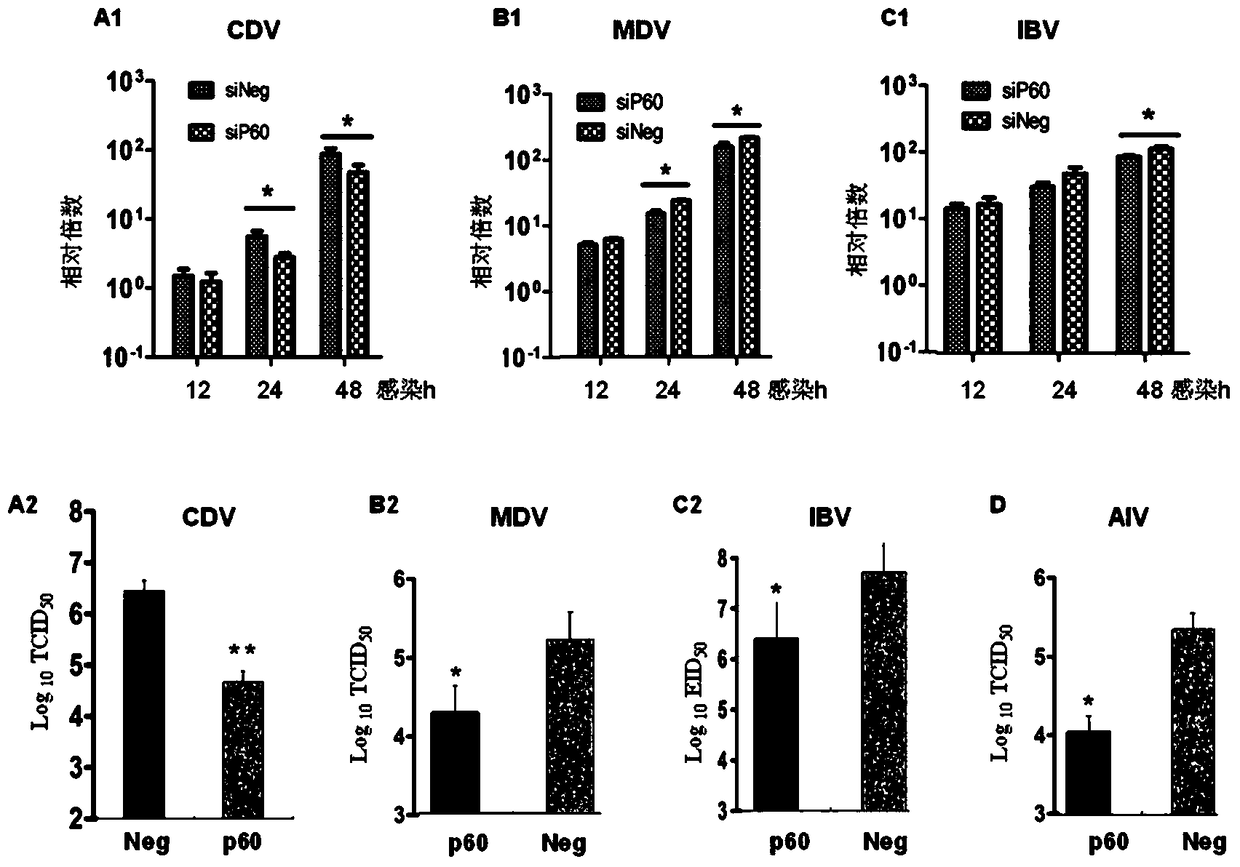
[0031] Example 1 Cell half infection dose and chick embryo half infection dose of several viruses
[0032] Take 9-11 day-old SPF chicken embryos to prepare chicken embryo fibroblasts (CEF), inoculate CDV, MDV and IBV on CEF and culture for 48 hours, harvest cells and culture supernatant repeatedly freeze-thaw 3 times, and re-inoculate on CEF cells Continue to cultivate, so pass down to 3 generations. Dilute the virus solution 10 times, and inoculate cells in 96-well plates or chicken embryos. CDV measured cell half infection dose (TCID 50 ), MDV uses the formation of cytopathic acne spots to measure TCID 50 , EID 50 , to determine the virulence of the 3 viruses. The results are shown in Table 1-Table 3.
[0033] Table 1 The influence of CDV cell half infection amount (indirect immunofluorescence method)
[0034]
[0035] Calculated according to the formula of Reed and Muench: distance ratio=(lesion rate higher than 50%-50) / (lesion rate higher than 50%-lesion rate lowe...
Embodiment 2
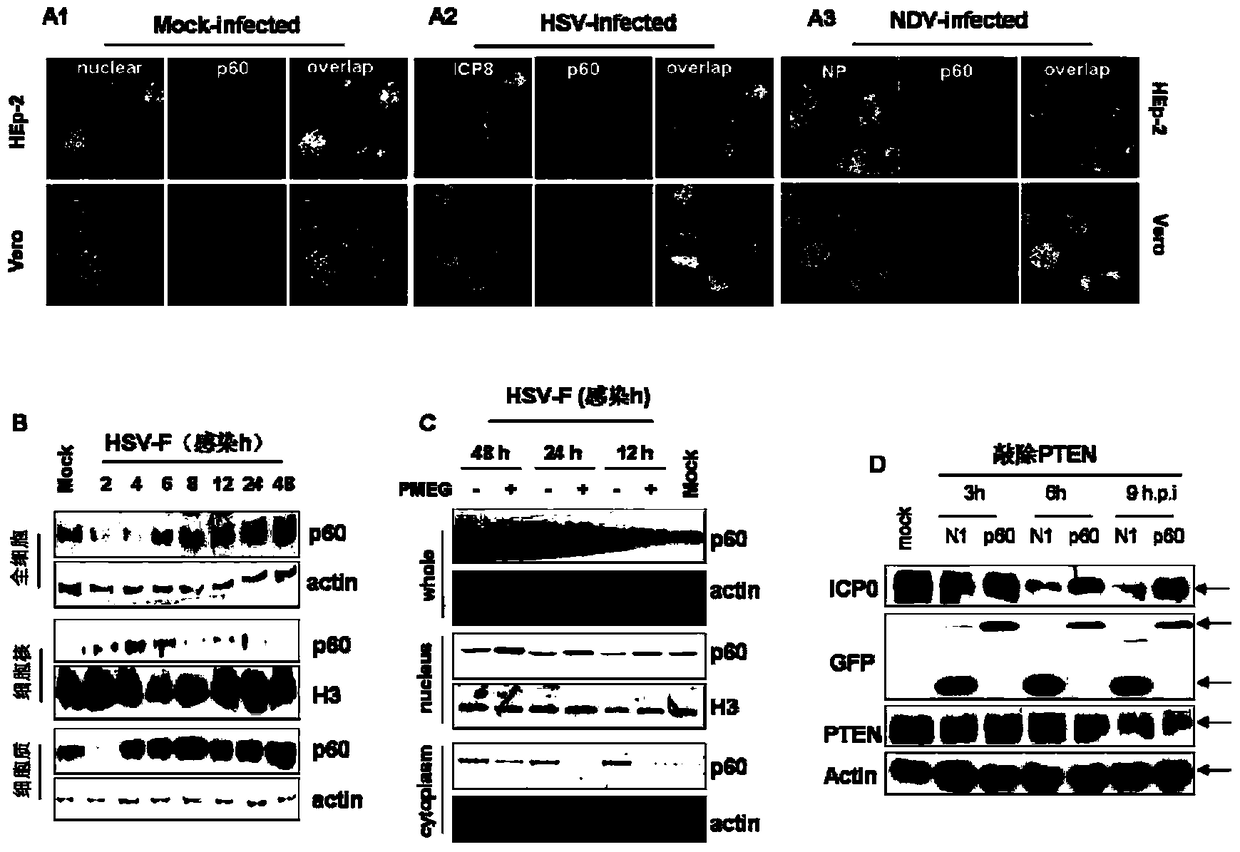
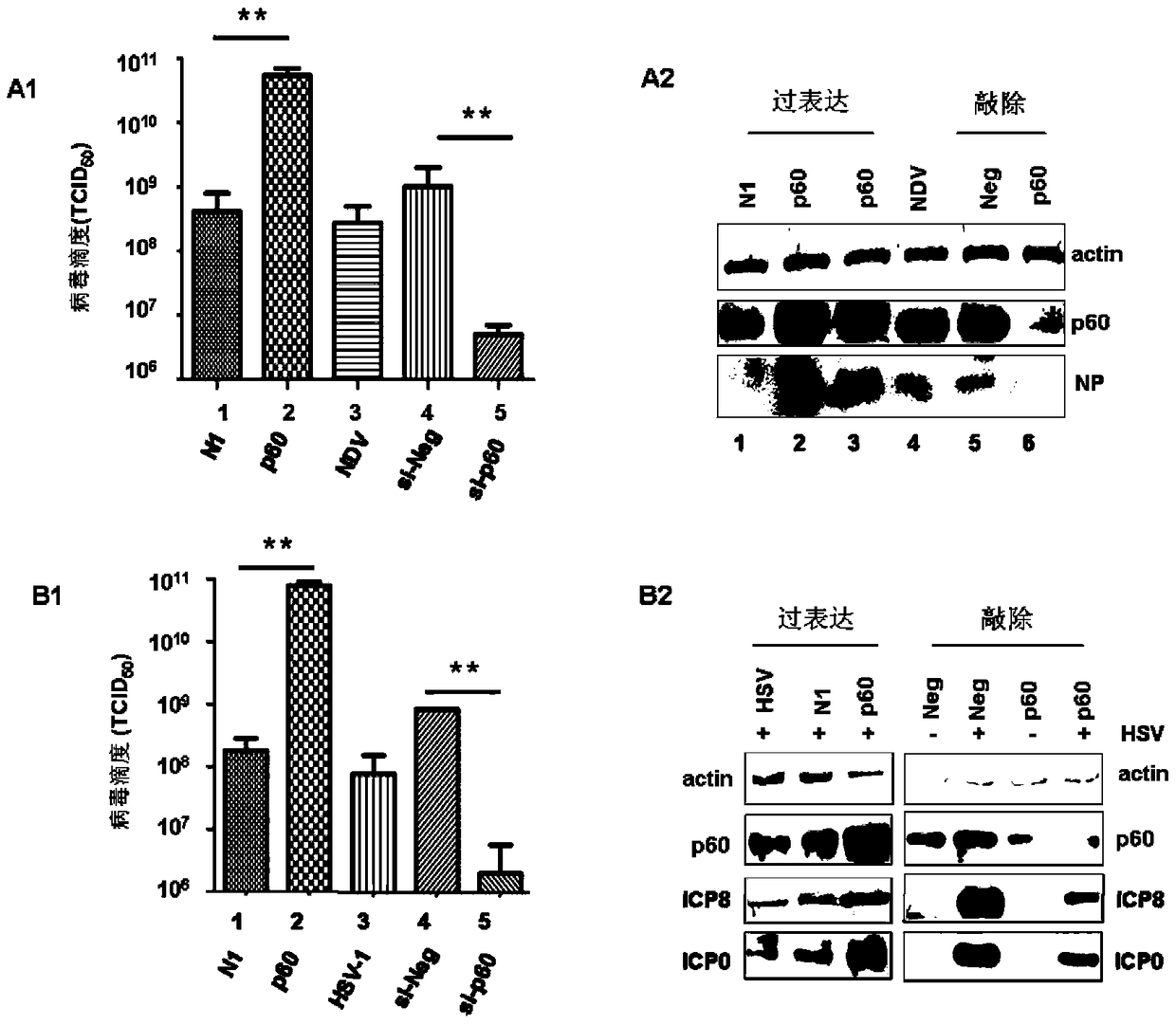
[0043] Example 2 Effect of knockout and overexpression of P60 on virus virulence (immunoblotting, lesion method and RT-PCR method)
[0044] 1. Cell preparation
[0045] Subculture Hep-2 and Vero cells: the growth medium contains 5% fetal bovine serum and 100U penicillin-streptomycin in DMEM, and the maintenance medium contains 2% fetal bovine serum and 100U penicillin-streptomycin in DMEM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com