Preparation method of parecoxib sodium synthesis technology impurities
A technology of parecoxib sodium and synthesis process, applied in the field of preparation of process impurity 4-benzenesulfonic acid ethyl ester, can solve the problems such as no impurity synthesis literature report and the like, achieve the effect of simple operation and control of finished product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
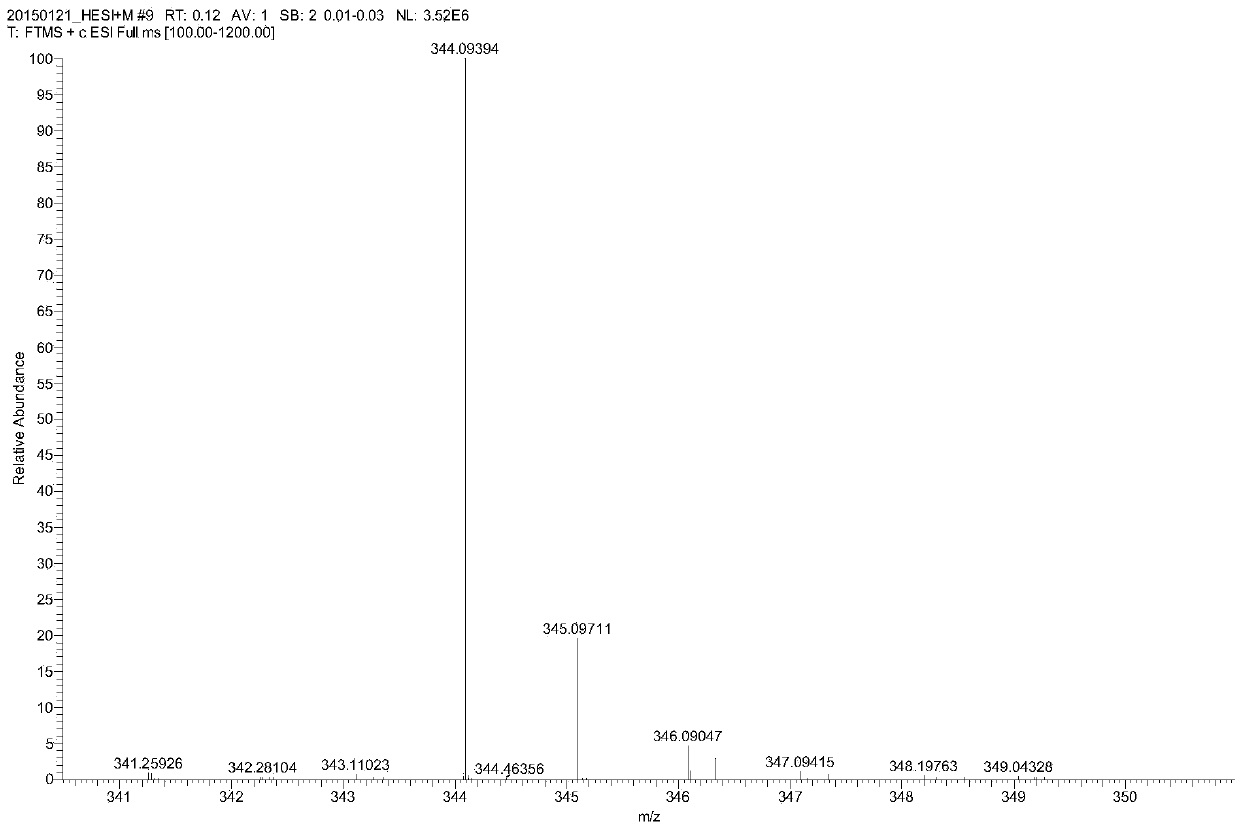
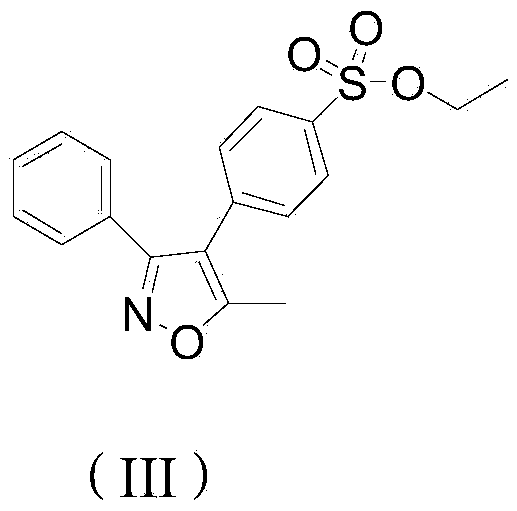
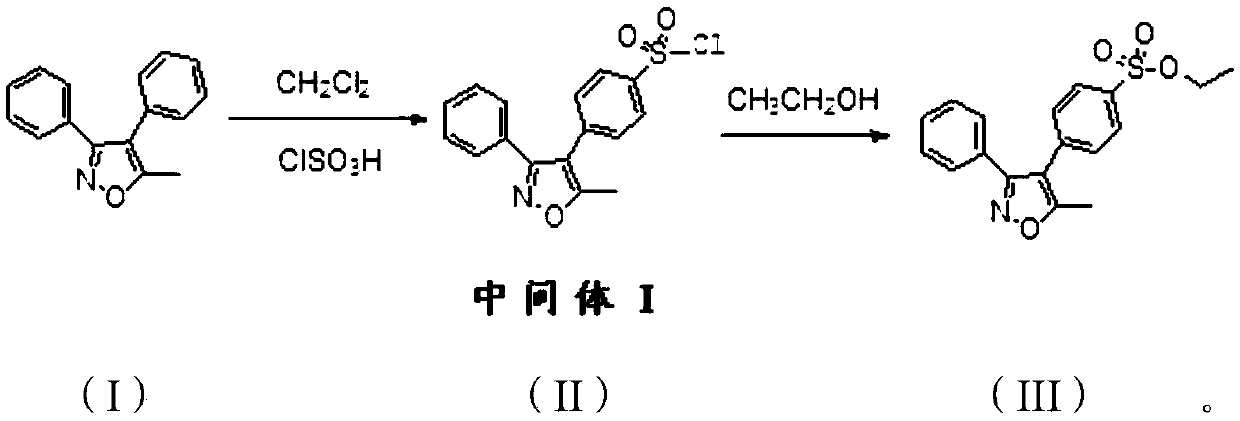
[0037] Example 1: A method for preparing parecoxib sodium synthesis process impurity 4-(5-methyl-3-phenyl-isoxazolyl) ethyl benzenesulfonate
[0038] Add 8.0 g of 5-methyl-3,4-diphenylisoxazole and 40 g of dichloromethane into the reaction flask, cool down to -5°C in an ice bath, start adding 32 g of chlorosulfonic acid dropwise, and control the internal temperature of the reaction solution to < 5 ℃. After the addition of chlorosulfonic acid was completed, the temperature was slowly raised to 35° C., and the reaction was kept for 10 hours. The reaction solution was monitored by TLC until the spot of the raw material 5-methyl-3,4-diphenylisoxazole disappeared (the developer was ethyl acetate:petroleum ether=1:6, v:v), and the reaction was stopped. Add 100 g of crushed ice to the reaction flask, stir for 1 h, pour into a separatory funnel and let stand to separate layers, and separate the lower aqueous phase. Add 5 g of anhydrous sodium sulfate to the upper organic phase, dry ...
Embodiment 2
[0044] Embodiment 2: A kind of synthetic method of parecoxib sodium process impurity 4-(5-methyl-3-phenyl-isoxazolyl) ethyl benzenesulfonate
[0045] Add 8.0 g of 5-methyl-3,4-diphenylisoxazole and 40 g of dichloromethane into the reaction flask, cool down to -5°C in an ice bath, start adding 40 g of chlorosulfonic acid dropwise, and control the internal temperature of the reaction solution to <5 ℃. After the addition of chlorosulfonic acid was completed, the temperature was slowly raised to 35° C., and the reaction was kept for 10 hours. The reaction solution was monitored by TLC until the spot of the raw material 5-methyl-3,4-diphenylisoxazole disappeared (the developer was ethyl acetate:petroleum ether=1:6, v:v), and the reaction was stopped. Add 100 g of crushed ice to the reaction flask, stir for 1 h, pour into a separatory funnel and let stand to separate layers, and separate the lower aqueous phase. Add 5 g of anhydrous sodium sulfate to the upper organic phase, dry i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com