Polysialic acid, blood group antigens and glycoprotein expression
A technology of antigens and oligosaccharides, applied in the direction of glycosyltransferases, enzymes, biochemical equipment and methods, etc., can solve the problems of expensive PEGylation process, molecular structure change, and biological activity reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0226] Plasmid construction
[0227] In this study, plasmids were constructed in yeast using standard homologous recombination (ShanksRM, CaiazzaNC, HinsaSM, ToutainCM, O'TooleGA: A Saccharomyces cerevisiae-based molecular toolkit for manipulating genes in Gram-negative bacteria, Applied Environmental Microbiology (ApplEnvironMicrobiol) 2006, 72(7):5027-5036). Plasmids were recovered from yeast and confirmed by transfer into E. coli strain DH5α via PCR and / or sequencing. The list below describes the plasmids constructed during the course of this study. The plasmid name is followed by the inserted gene / sequence in order from 5' to 3', followed by the vector in brackets. Glycan expression plasmids were constructed in vector pMW07 (Vaderrama-Rincon et al.). Protein expression plasmids are typically constructed in the vector pTRCY. The sugar nucleotide synthesis plasmid was cloned in pTrcY, pMQ70.
[0228] In the order of the pictures:
[0229] pMW07: (Vector) pBAD, ChlorR, ...
Embodiment 2
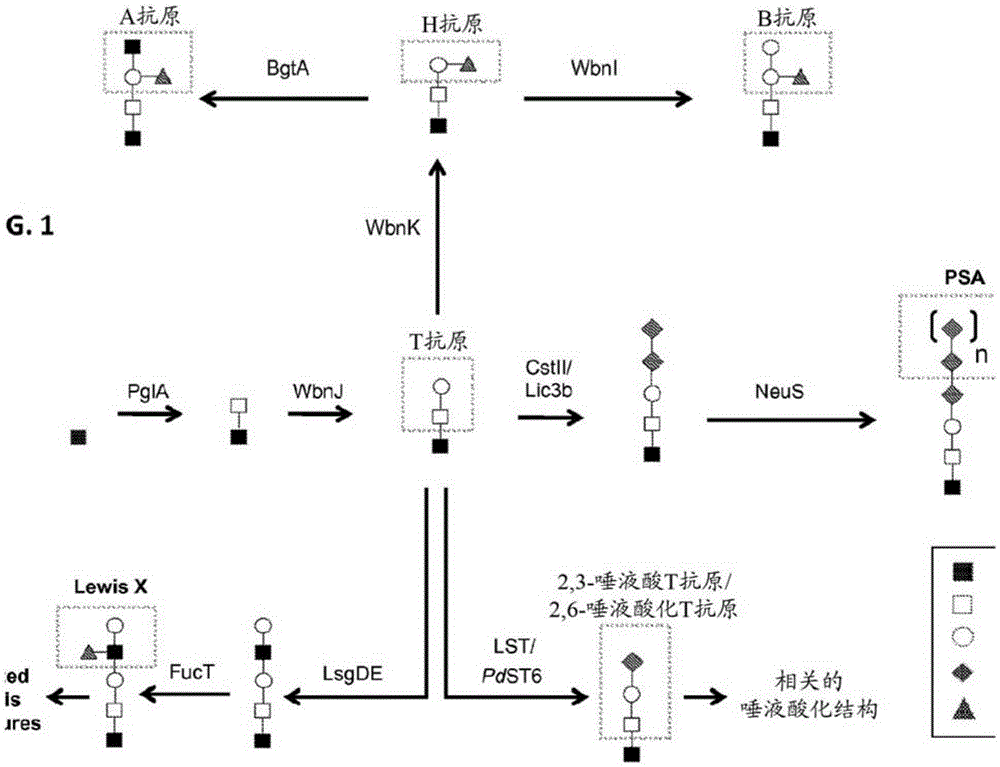
[0271] Engineered Escherichia coli for expression of human Thomsen-Friedenreich antigen (T antigen)
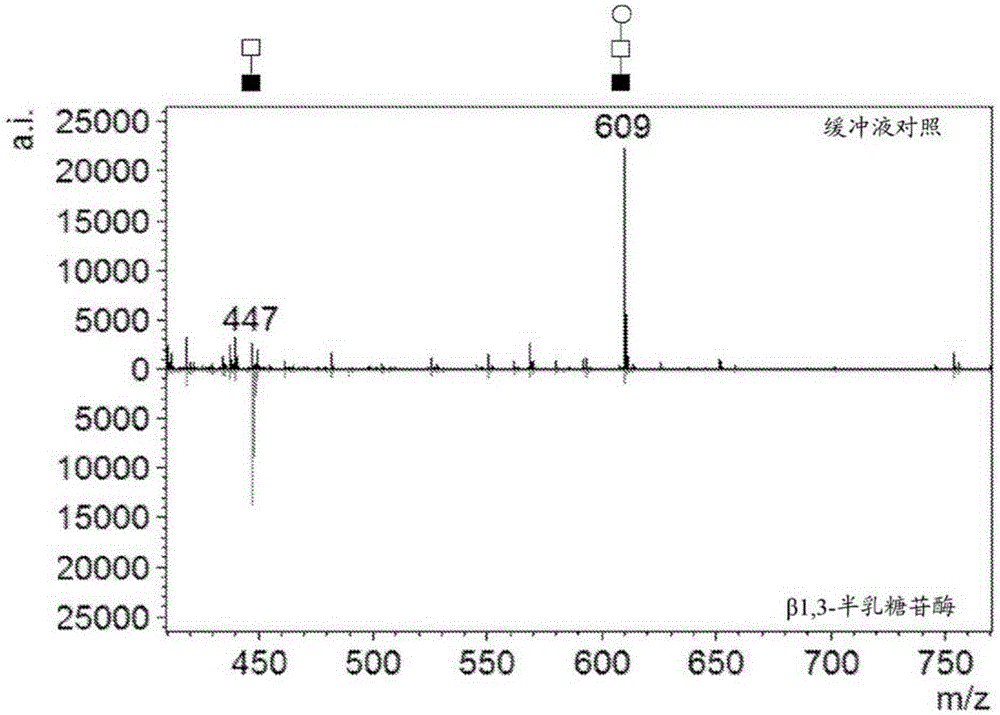
[0272] T-antigen glycans (T-antigen, Galβ1,3GalNAcα-) are structures found at the core of many human-related human glycans. Construction of a plasmid for the production of glycosyltransferase and sugar nucleotide epimers necessary for constructs using native UndPP-GlcNAc as substrate for the assembly of human T-antigen-containing glycans in E. coli Expression of enzyme activity. Plasmid pMW07 (Valderrama-Rincon et al.) was used as a vector because it contains a low copy number origin of replication (ORI), an inducible pBAD promoter, and allows cloning in Saccharomyces cerevisiae via homologous recombination Yeast ORI. The sequence of pMW07 is provided as SEQ ID NO:1.
[0273] To generate disaccharide glycans with the GalNAcα1,3GlcNAc structure, a plasmid was constructed to express the Campylobacter jejuni GalNAc transferase PglA and the epimerase GalE to facilitate the synt...
Embodiment 3
[0280] In vivo synthesis of proteins bearing V-glycans terminated in human T antigens
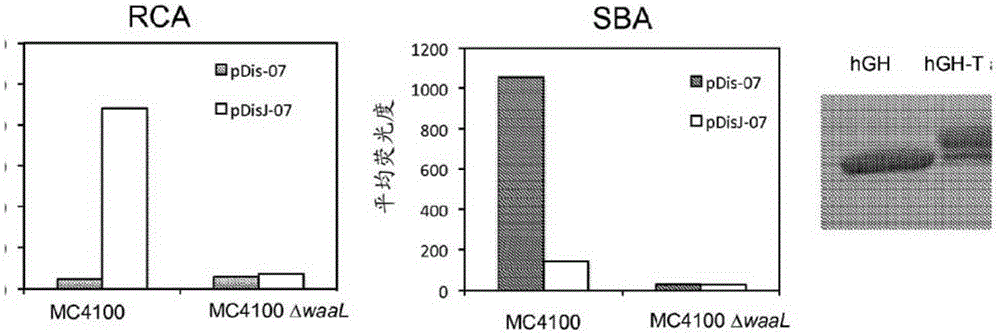
[0281] Transfer of UndPP-linked oligosaccharides to specific asparagine residues using OSTPglB. This requires a D / EX1NX 2 The target protein consisting of the S / T sequon to be localized into the periplasm, bearing the PglB recognition site, and the presence of an appropriate glycan substrate. In this study, we also constructed the vector pTRCY for glycoprotein expression.
[0282] pTRCY was cloned in S. cerevisiae via homologous recombination by adding the URA3 gene and yeast 2 micron ORI to pTRC99a, resulting in a novel vector capable of replicating in yeast. The URA3 gene and the 2 micron ORI were amplified using primers containing homology to pTRC99a for insertion between the pBR322 ORI and the lacI gene. The sequence of vector pTRCY is SEQ ID NO:6.
[0283] hGH was cloned as a C-terminal translational fusion following the signal peptide, MBP, hexahistidine tag, and tev cleavage site...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com