Lyophilized orally disintegrating tablets of trelagliptin succinate
A technology of trelagliptin succinate and orally disintegrating tablet is applied in the field of freeze-dried orally disintegrating tablet of trilagliptin succinate and its preparation field, which can solve the complex preparation process and slow oral disintegration of ordinary oral disintegrating tablet. , poor compliance and other problems, to achieve the effect of improving taste and disintegration time, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
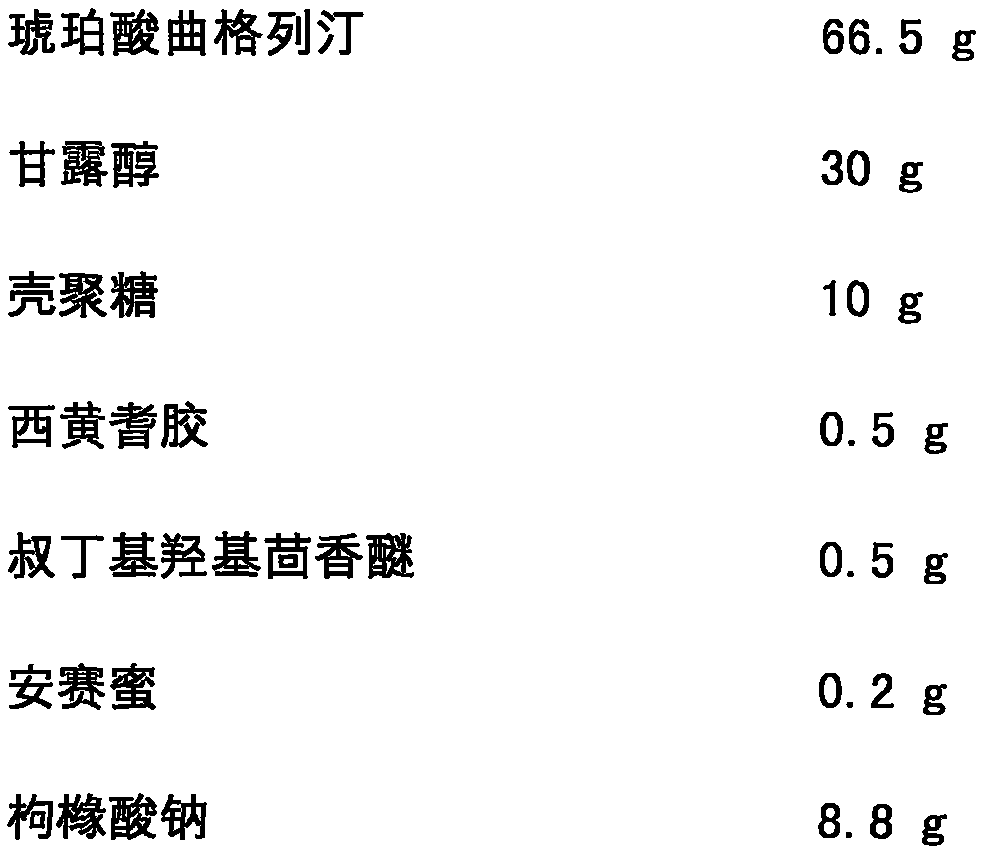
[0027] Trexagliptin succinate freeze-dried orally disintegrating tablets are prepared from the following components, and the dosage is 1000 tablets:
[0028]
[0029] Its preparation method comprises the following steps:
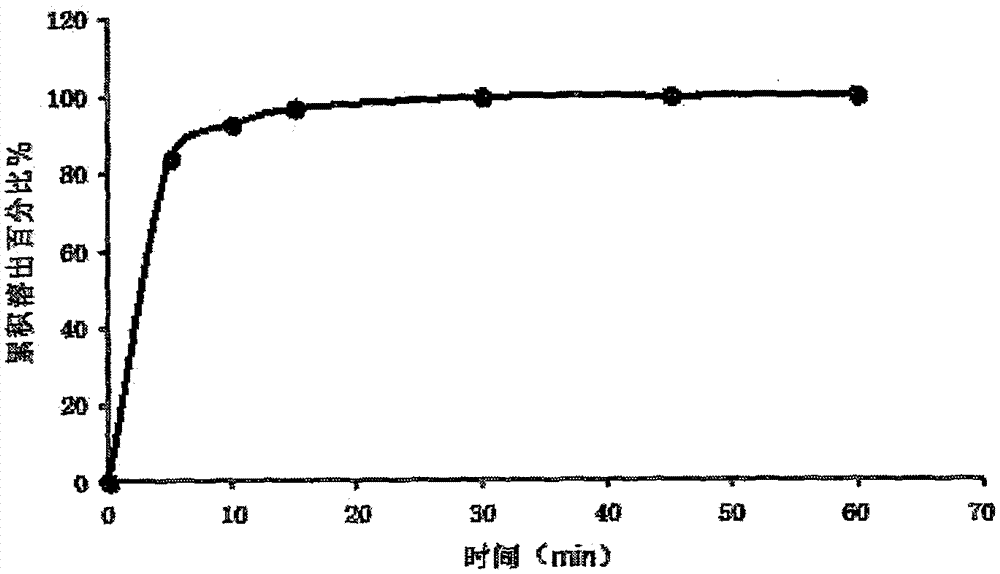
[0030] The specific preparation method is as follows: mix troxagliptin succinate, mannitol, chitosan, tragacanth gum, tert-butyl hydroxyanisole, acesulfame potassium and sodium citrate, and add prescription Mix a certain amount of purified water to make a uniform solution; vacuum degas the solution and inject it into the mold accurately; after pre-freezing for 1-60 minutes, transfer it to a lyophilizer and freeze-dry for 1-10 hours to obtain the amber of the present invention Trexagliptin Orally Disintegrating Tablets. The taste is not bitter, no gritty feeling, and the oral disintegration time is 20s.
Embodiment 2
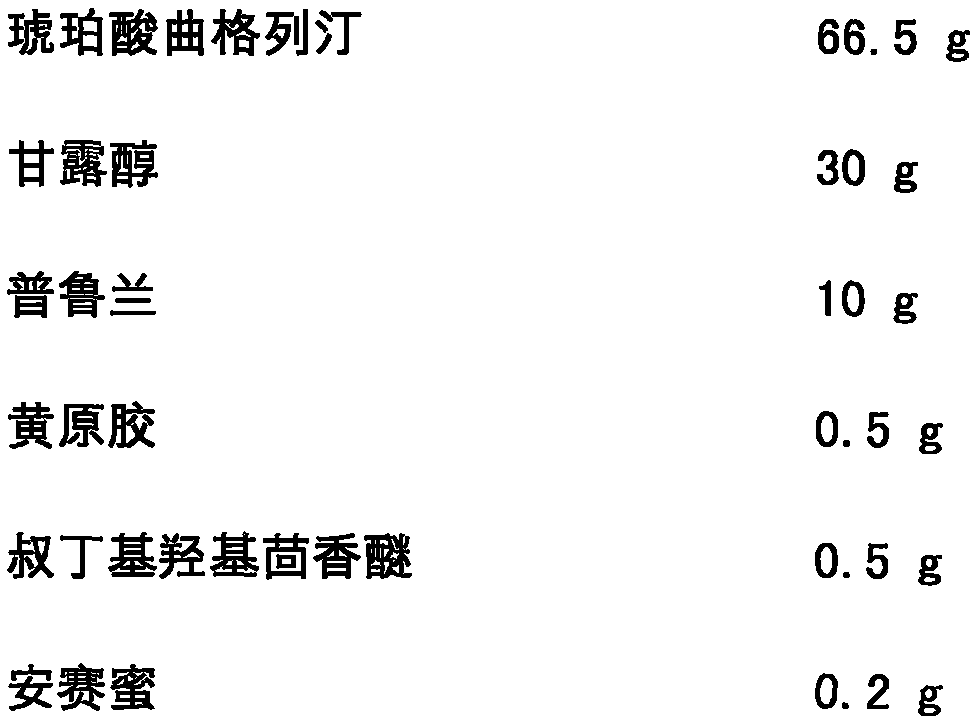
[0032] Trexagliptin succinate freeze-dried orally disintegrating tablets are prepared from the following components, and the dosage is 1000 tablets:
[0033]
[0034]
[0035] Its preparation method comprises the following steps:
[0036] The specific preparation method is as follows: mix troxagliptin succinate, mannitol, pullulan, xanthan gum, tert-butyl hydroxyanisole, acesulfame potassium and sodium citrate evenly, and add the prescribed amount of purified water, mixed to make a homogeneous solution; after the solution is vacuum degassed, it is accurately injected into a mold; after pre-freezing for 1-60 minutes, it is transferred to a freeze dryer, and freeze-dried for 1-10 hours to obtain the succinic acid of the present invention Trexagliptin orally disintegrating tablets. The taste is not bitter or gritty, and the oral disintegration time is 22s.
Embodiment 3
[0038] Trexagliptin succinate freeze-dried orally disintegrating tablets are prepared from the following components, and the dosage is 1000 tablets:
[0039]
[0040] Its preparation method comprises the following steps:
[0041] The specific preparation method is as follows: mix troxagliptin succinate, mannitol, pullulan, xanthan gum, tert-butyl hydroxyanisole, acesulfame potassium and sodium citrate evenly, and add the prescribed amount of purified water, mixed to make a homogeneous solution; after the solution is vacuum degassed, it is accurately injected into a mold; after pre-freezing for 1-60 minutes, it is transferred to a freeze dryer, and freeze-dried for 1-10 hours to obtain the succinic acid of the present invention Trexagliptin orally disintegrating tablets. The taste is sweet, without gritty feeling, and the oral disintegration time is 27s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com