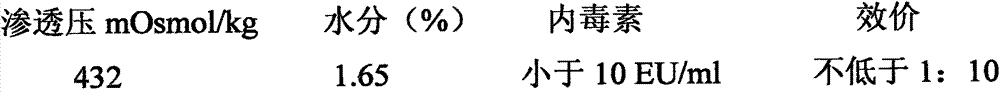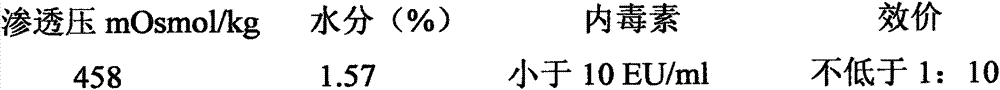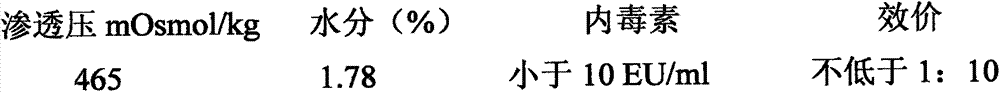Gelatin-free bivalent renal syndrome vaccine freeze-drying protective agent
A technology of jelly and gelatin, which is applied in the direction of inorganic non-active ingredients, medical preparations of non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as uncertain ingredients, allergic reactions, and gelatin that can easily cause allergic reactions, etc. , to achieve the effect of reducing the harm of the incidence of side effects and increasing the safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] preparation of dilutions,
[0043] Put raw material A in the diluent formula in a beaker, add diluent D and stir to dissolve at a stirring speed of 100 rpm for 1 minute, then add raw material B and raw material C and stir to dissolve at a stirring speed of 100 rpm Minutes, stirring for 2 minutes, after that, add diluent D to make the total volume constant to 1000 ml, stir and mix, stirring speed 100 rpm, stirring for 5 minutes, after that, adjust the pH value with 20% pharmaceutical grade hydrochloric acid Adjust to 7.4±0.1, sterilize with moist heat, obtain diluent, stand-by;
Embodiment 1
[0045] Product name: Gelatin-free Double Kidney Seedling Freezing Preservative-Type 1
[0046] formula:
[0047] Raw material 1: sucrose, pharmaceutical grade, 50 grams;
[0048] Raw material 2: glycine, analytically pure, 25 grams;
[0049] Raw material 3: human serum albumin, biological products, 0.2 g;
[0050] Raw material 4: arginine, analytically pure, 2 grams;
[0051] Raw material 5: mannitol, analytically pure, 2 grams;
[0052] Diluent: Phosphate buffer saline PBS, pH7.4±0.1, balance, ml;
[0053] The formula unit is the material ratio of 1000 ml unit capacity;
[0054] Preparation Process:
[0055] Step 1. Dissolve raw material 1, raw material 2, raw material 4 and raw material 5 in the gel-free cryoprotectant formula in 200 ml of the diluent in a beaker, stir to dissolve, and stir at a stirring speed of 100 rpm. It takes 2 minutes, after that, add raw material 3, stir and mix, the stirring speed is 100 rpm, and it takes 5 minutes to stir, after that, add the...
Embodiment 2
[0058] Product name: Gelatin-free double kidney seedling cryopreservative-type 2
[0059] formula:
[0060] Raw material 1: sucrose, pharmaceutical grade, 50 grams;
[0061] Raw material 2: glycine, analytically pure, 45 grams;
[0062] Raw material 3: human serum albumin, biological products, 0.2 g;
[0063] Raw material 4: arginine, analytically pure, g;
[0064] Raw material 5: mannitol, analytically pure, g;
[0065] Diluent: Phosphate buffer saline PBS, pH7.4±0.1, balance, ml;
[0066] The formula unit is the material ratio of 1000 ml unit capacity;
[0067] Preparation Process:
[0068]Step 1. Dissolve raw material 1, raw material 2, raw material 4 and raw material 5 in the gel-free cryoprotectant formula in 200 ml of the diluent in a beaker, stir to dissolve, and stir at a stirring speed of 100 rpm. It takes 2 minutes, after that, add raw material 3, stir and mix, the stirring speed is 100 rpm, and it takes 5 minutes to stir, after that, add the diluent to make t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com