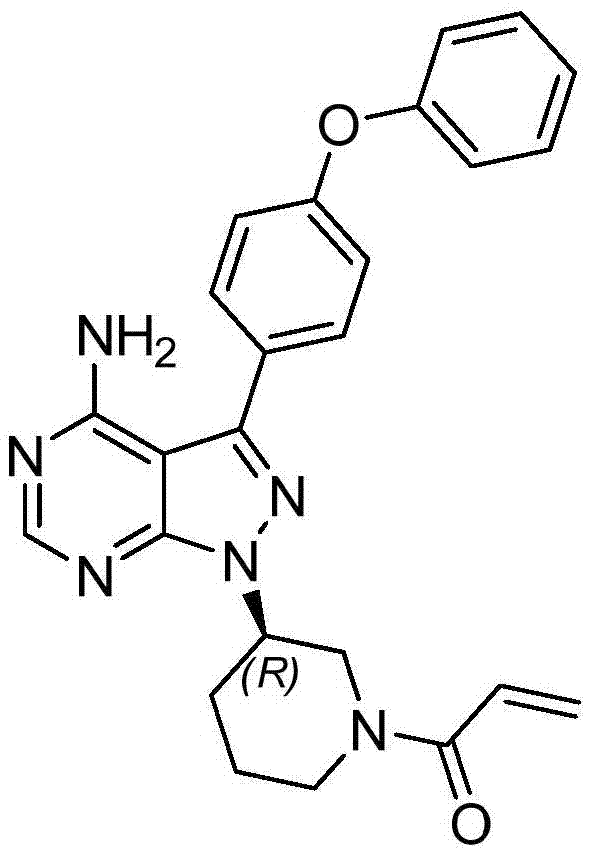
Ibrutinib purification method
A technology of ibrutinib and purification method, applied in the field of compound purification, can solve the problems of long operation time, cumbersome operation, high operator requirements, etc., and achieve the effect of simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
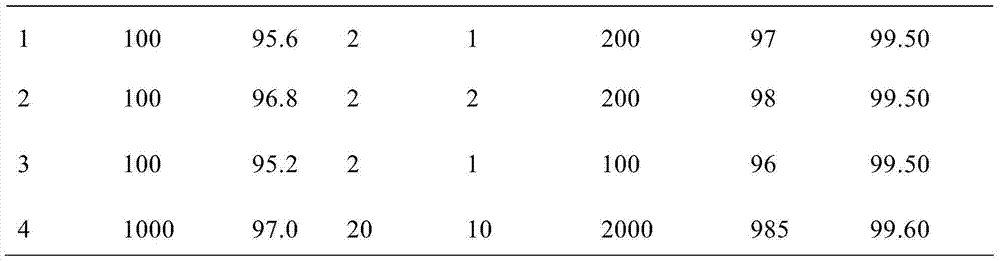
[0025] Embodiment 1~4 Silica gel adsorption purification Lutinib crude product
[0026] Put the crude ibrutinib into the reaction bottle, add dichloromethane, stir to dissolve, add 200-300 mesh silica gel for column chromatography, raise the temperature to 30-40°C, stir for half an hour, filter while hot, and use the filter cake Rinse with ethyl acetate at 70-77°C, and concentrate the obtained filtrate to dryness to obtain the semi-finished product of ibrutinib.
[0027] Examples 1-4 were purified by column chromatography with silica gel adsorption to obtain semi-finished products of ibrutinib.
[0028] Table 1 Silica gel adsorption purification parameter table
[0029]
[0030]
Embodiment 5~11
[0033] Put the semi-finished product of ibrutinib into the reaction bottle, add a medium-strong polar organic solvent to raise the temperature to reflux, stir until clarification, add the anti-solvent dropwise, after the addition is completed, stir at T°C for half an hour, cool down to crystallize, and cool down to 0-20 °C, filter and dry to obtain the finished product of ibrutinib. The specific parameters are shown in Table 2.
[0034] Table 2 Examples 5-11 Ibrutinib Semi-finished Product Recrystallization Purification Parameters
[0035]
[0036]
[0037] In Examples 5-11, the semi-finished ibrutinib was used as the raw material to obtain the finished ibrutinib through further recrystallization and purification. The results are shown in Table 2, the purity of ibrutinib finished products were all increased to 99.8% and above.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com