Method for highly selectively detecting concentration of hydrazine
A technology for concentration and sample detection, applied in chemical instruments and methods, measuring devices, instruments, etc., can solve the problems of complex synthesis, poor selectivity, low sensitivity, etc., and achieve the effect of simple synthesis and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] (Scheme 1) 4-Hydroxystyrenetricyanofuran (303mg, 1.0mmol), 4-bromobutyric acid (166mg, 1.0mmol), 4-dimethylaminopyridine (122mg, 1.0mmol) and dicyclohexyl carbon Diimine (412 mg, 2.0 mmol) was dissolved in 20 mL of dry dichloromethane, reacted at 50 °C for 4 h, evaporated under reduced pressure to obtain a crude product, and then separated by column chromatography using dichloromethane to obtain 140 mg of a light yellow pure product. The yield was 31%.
[0026] (Scheme 2) 4-Hydroxystyrenetricyanofuran (303mg, 1.0mmol), 4-bromobutyric acid (166mg, 1.0mmol), 4-dimethylaminopyridine (122mg, 1.0mmol) and dicyclohexyl carbon Diimine (412 mg, 2.0 mmol) was dissolved in 20 mL of dry dichloromethane, reacted at 50 °C for 10 h, evaporated under reduced pressure to obtain a crude product, and then separated by column chromatography using dichloromethane to obtain 293 mg of a light yellow pure product. The yield was 65%.
[0027] (Scheme 3) 4-Hydroxystyrene tricyanof...
Embodiment 2
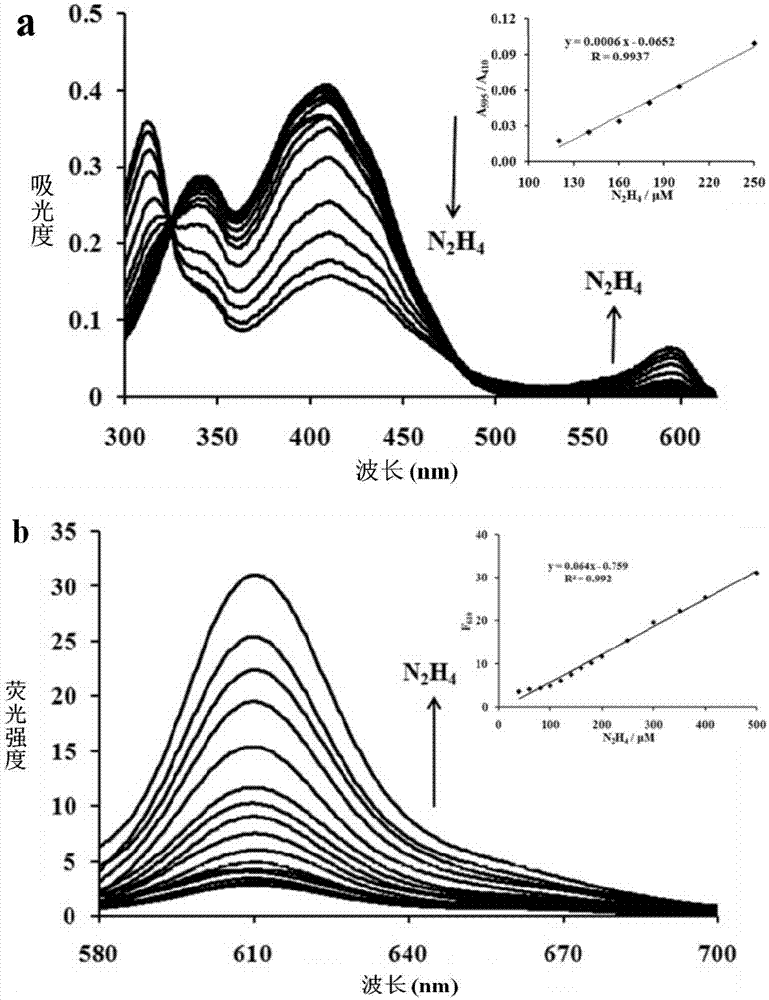
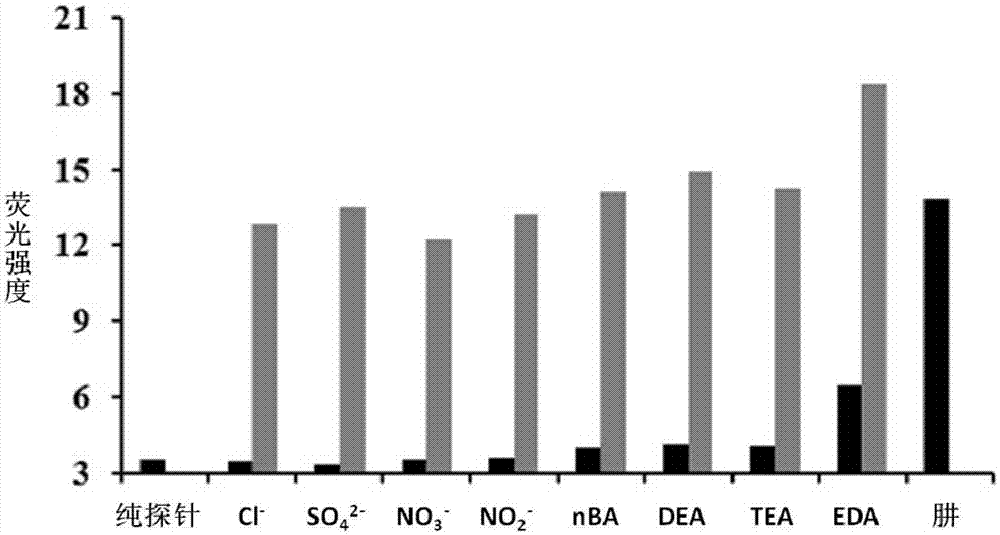
[0031] The inventor of the present invention has carried out following test: (a) the impact of different concentrations of hydrazine (0~500 μ M) on the absorption spectrum of probe (20 μ M), insert figure is the ratio of the absorbance at 595nm place and the absorbance at 410nm place and the concentration of hydrazine added linear relationship between them. (b) Effects of different concentrations of hydrazine (0-500 μM) on the fluorescence spectrum of the probe (20 μM); the insert graph is the linear relationship between the fluorescence intensity at 610 nm and the concentration of added hydrazine. The above determinations were made in a system containing ethanol and water (5:5, V / V) containing 5mM phosphate buffered saline (PBS) with a pH value of 7.4, and all spectral tests were performed at 25°C after adding hydrazine for 20 minutes. of. See results figure 1 .
[0032] From figure 1 a It can be seen that with the increase of the concentration of hydrazine in the probe s...
Embodiment 3
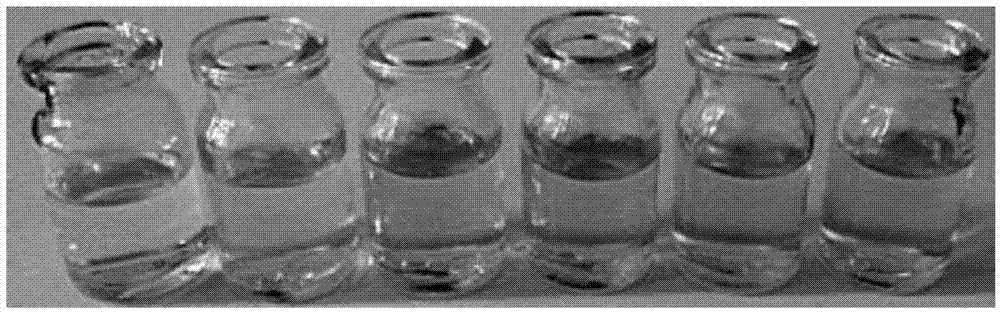
[0034] Depend on figure 2 It can be clearly seen that the color of the probe solution (40 μM) gradually changes from yellow to blue in the presence of different concentrations of hydrazine (from left to right: 0, 0.2, 0.4, 0.6, 0.8, 1.0 mM). Therefore, the probe of the present invention can complete the qualitative and quantitative analysis and detection of hydrazine in the sample only by "naked eye" observation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com