Triterpenoid saponins compound, and preparation method and uses thereof
A technology of triterpene saponins and compounds, which is applied in the field of medicine, can solve the problems that the structure of natural ginsenosides is not in the best state, has not been developed and utilized, and achieves obvious anti-tumor activity and good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] One, a kind of triterpene saponin compound structural formula that the present invention relates to is as follows:
[0044]
[0045] Its chemical name is: 6-O-β-D-glucoside-Dama-22,(23)(Z)-ene-3β,6α,12β,20,25-pentaol-20-furan ring side chain. The triterpene saponins compound described in the present invention is a new compound isolated for the first time from the transformation product of Panax notoginseng, named pseudo-ginsenoside RT 7 .
[0046] The physicochemical constants of triterpene saponins compound described in the present invention are as follows:
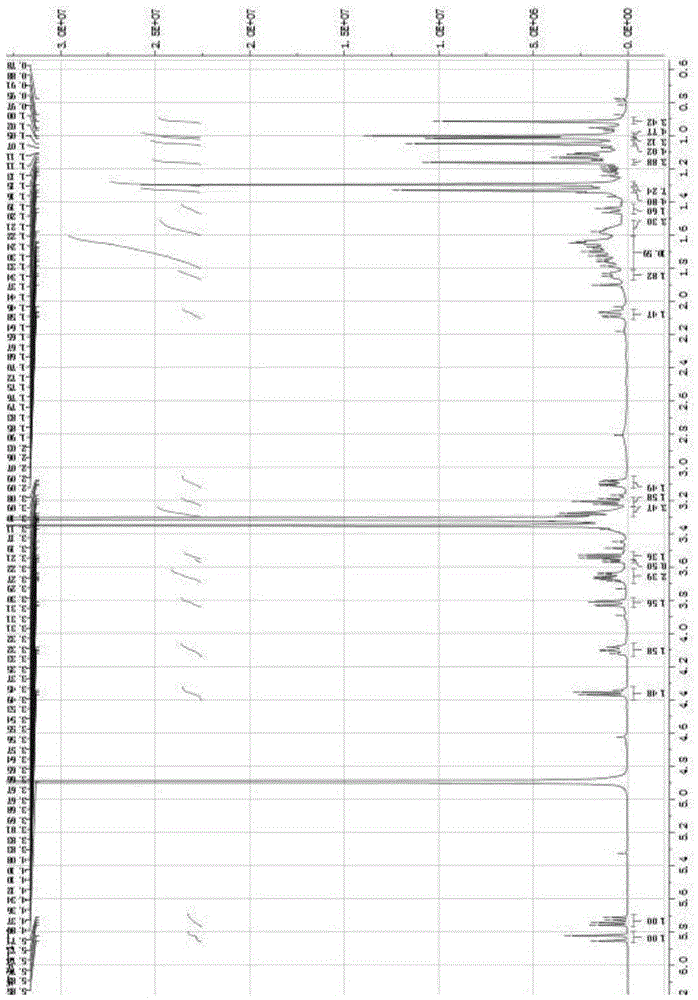
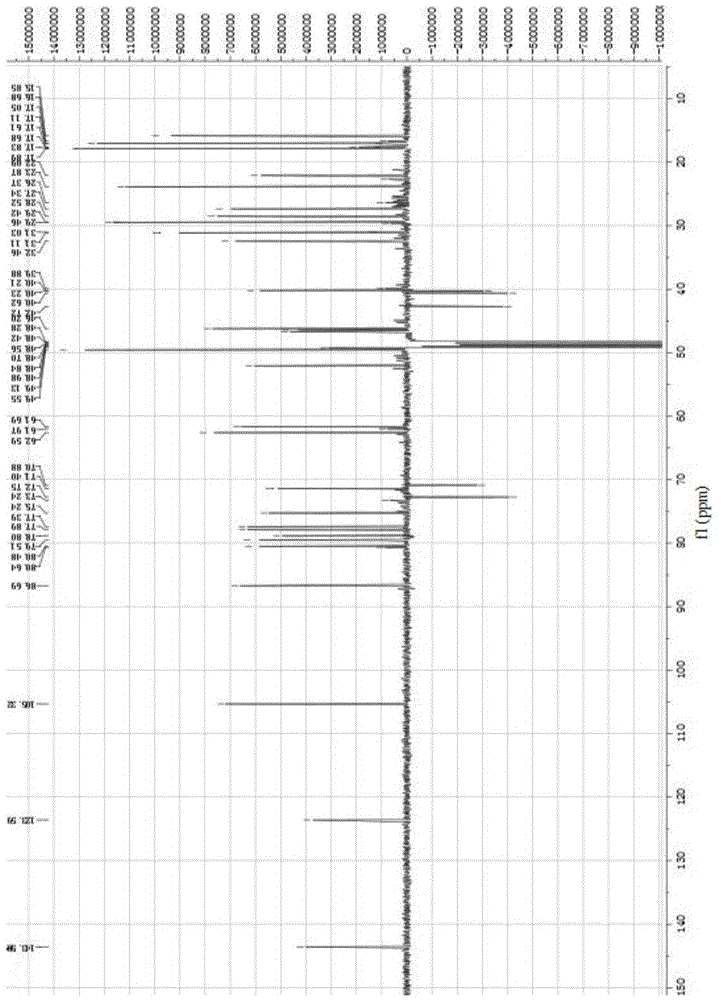
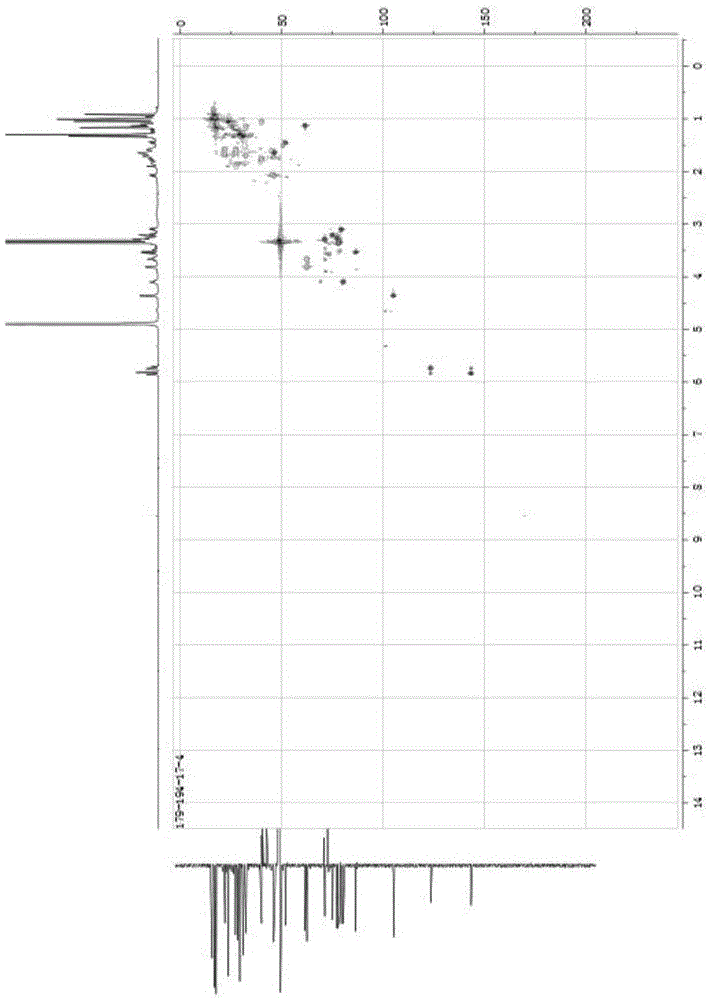
[0047] White powder (methanol), ESI-MS: m / z675.40 ([M+Na] + ), m / z687.38 ([M+Cl] – ), combined with hydrogen spectrum and carbon spectrum, it is speculated that its molecular formula is C 36 h 60 o 10 . 10% sulfuric acid ethanol solution was purple, Molish reaction was positive, Liebermann-Burchard reaction was positive. 1 H and 13 See Table 1 for C-NMR data.
[0048] Table 1 of compounds 1 H-NMR, 1...
Embodiment 2
[0070] Embodiment 2: Antitumor activity test of the compound of the present invention
[0071] 1. Test materials
[0072] 1. Tumor strains tested in vitro:
[0073] Helas human cervical cancer cells were provided by the Pharmacology Laboratory of the R&D Center of Changchun University of Traditional Chinese Medicine.
[0074] 2. Reagents for in vitro tests:
[0075] Newborn bovine serum, dimethyl sulfoxide (DMSO) and MTT tetraoxalate (Sigma).
[0076] 3. In vitro test equipment:
[0077] Microplate reader: Multiskan Ascent, model: VL.23354-00541T.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com