Triterpenoid saponin compound and preparation method and application thereof
A technology for triterpenoid saponins and compounds, applied in the field of medicine, can solve the problems that have not been developed and utilized, and the structure of natural ginsenosides is not in the optimal state of structure, and achieve the effect of obvious anti-tumor activity and good anti-tumor effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] One, a kind of triterpene saponin compound structural formula that the present invention relates to is as follows:
[0044]
[0045] Its chemical name is: said triterpenoid saponins compound is the new compound that is isolated from the notoginseng conversion product for the first time, named ginsenoside: 6-O-β-D-glucopyranosyl-Dama-17α (H )-20(22)(E)-ene-3β,6α,12β,24S,25-pentaol. The name of the triterpene saponins compound of the present invention is ginsenoside ST-7.
[0046] The physicochemical constants of triterpene saponins compound described in the present invention are as follows:
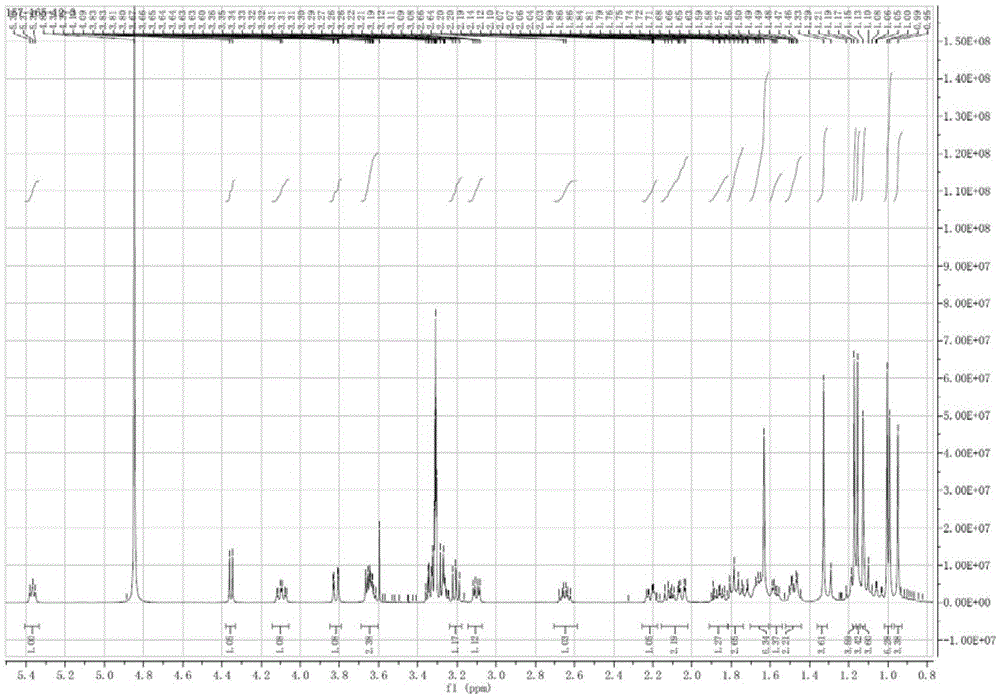
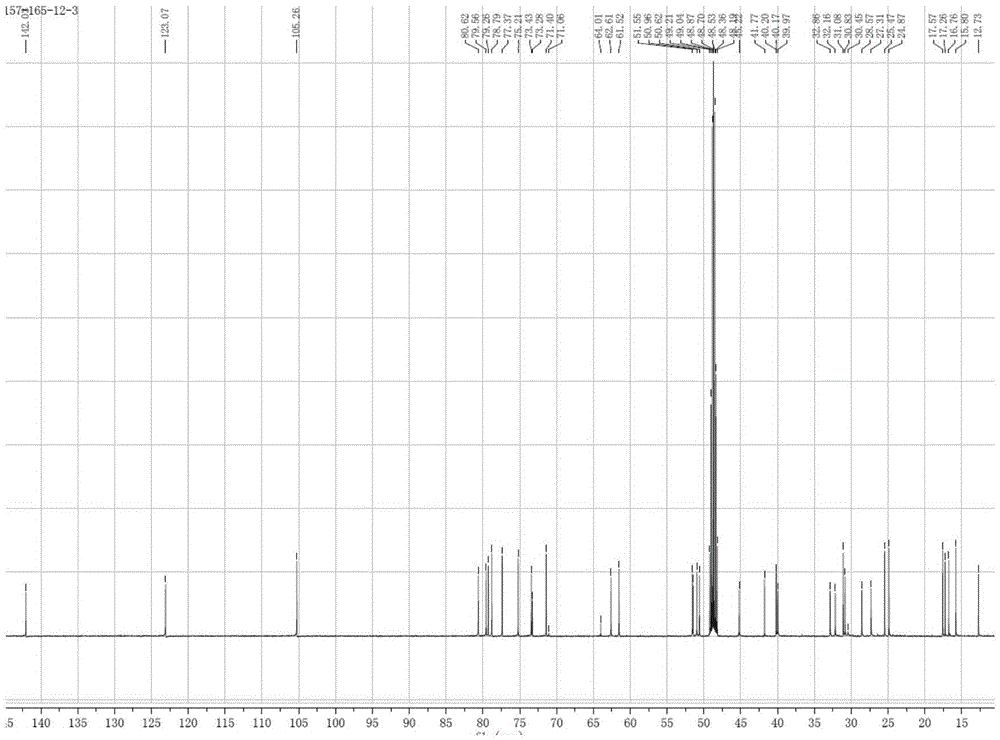
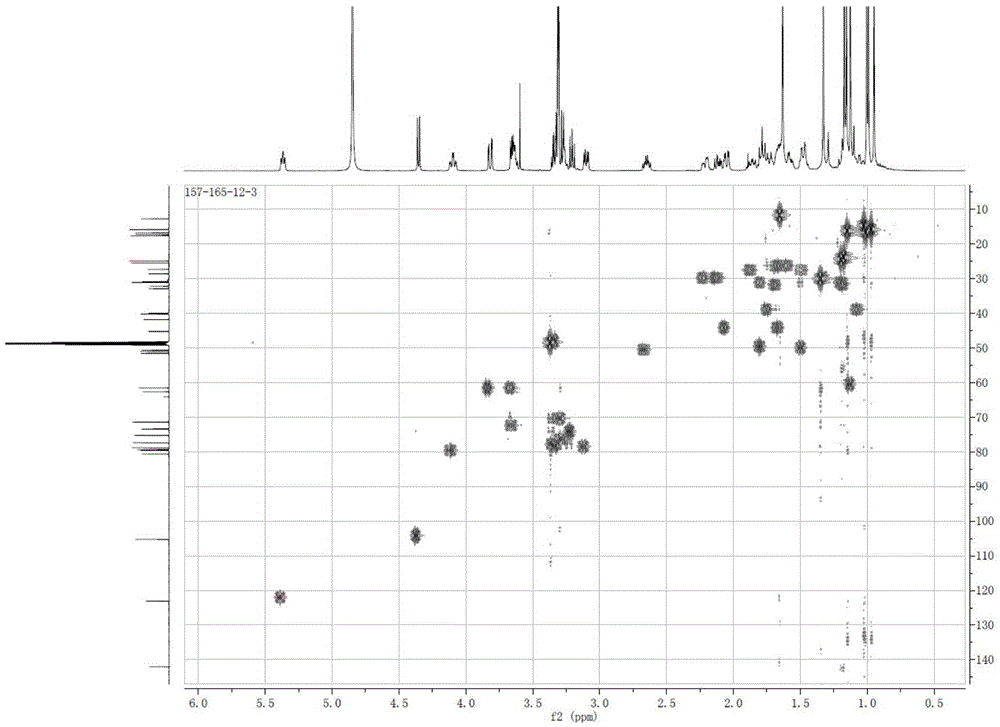
[0047] White powder (methanol), ESI-MS: m / z677.42 ([M+K] + ), combined with hydrogen spectrum and carbon spectrum, it is speculated that its molecular formula is C 36 h 62 o 9 . 10% sulfuric acid ethanol solution was purple, Molish reaction was positive, Liebermann-Burchard reaction was positive. 1 H and 13 See Table 1 for C-NMR data.
[0048] 1H-NMR, 13C-NMR data attrib...
Embodiment 2
[0070] Embodiment 2: Antitumor activity test of the compound of the present invention
[0071] 1. Test materials
[0072] 1. Tumor strains tested in vitro:
[0073] Helas human cervical cancer cells were provided by the Pharmacology Laboratory of the R&D Center of Changchun University of Traditional Chinese Medicine.
[0074] 2. Reagents for in vitro tests:
[0075] Newborn bovine serum, dimethyl sulfoxide (DMSO) and MTT tetraoxalate (Sigma).
[0076] 3. In vitro test equipment:
[0077] Microplate reader MultiskanAscent model VL.23354-00541T.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com