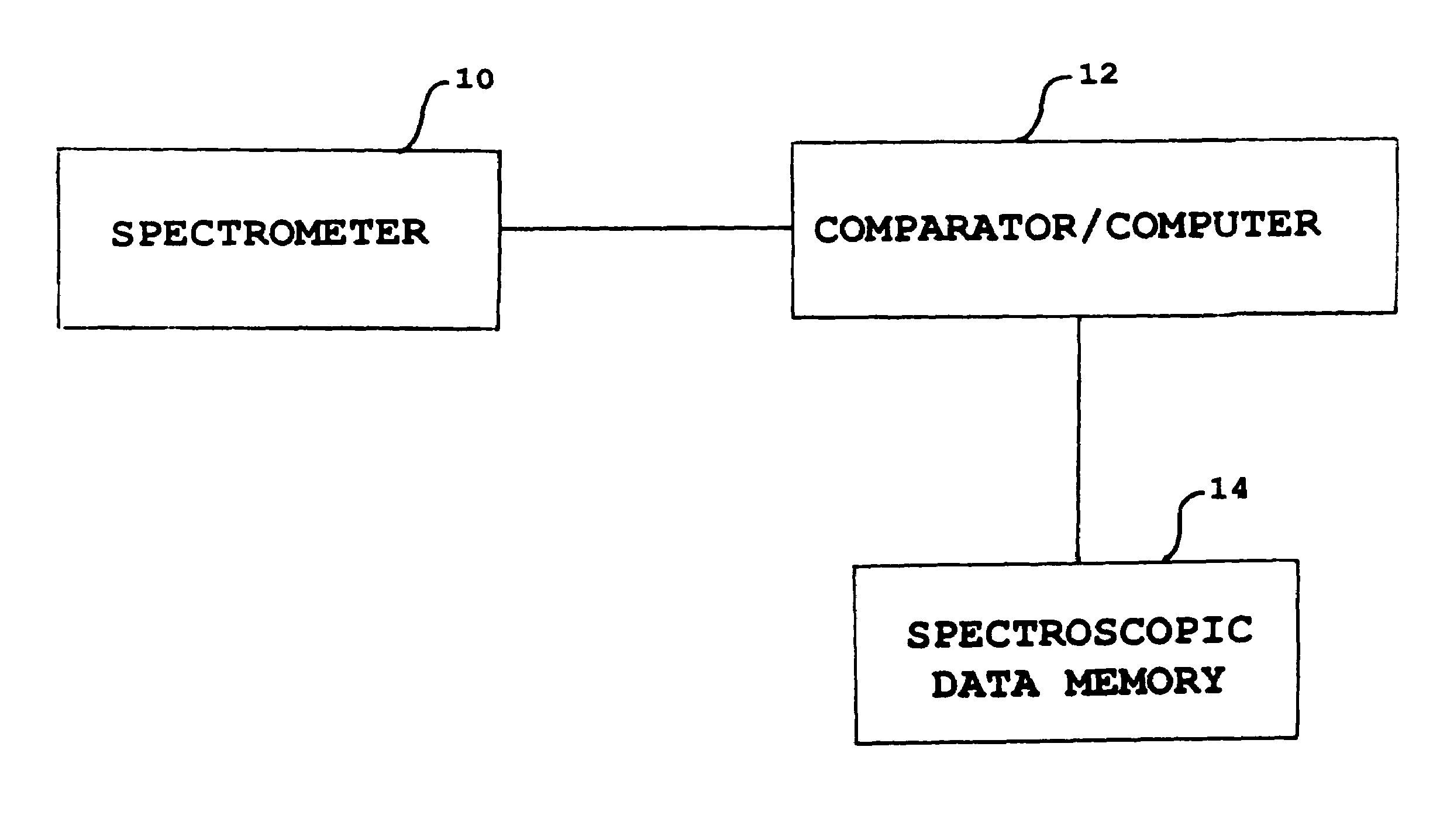
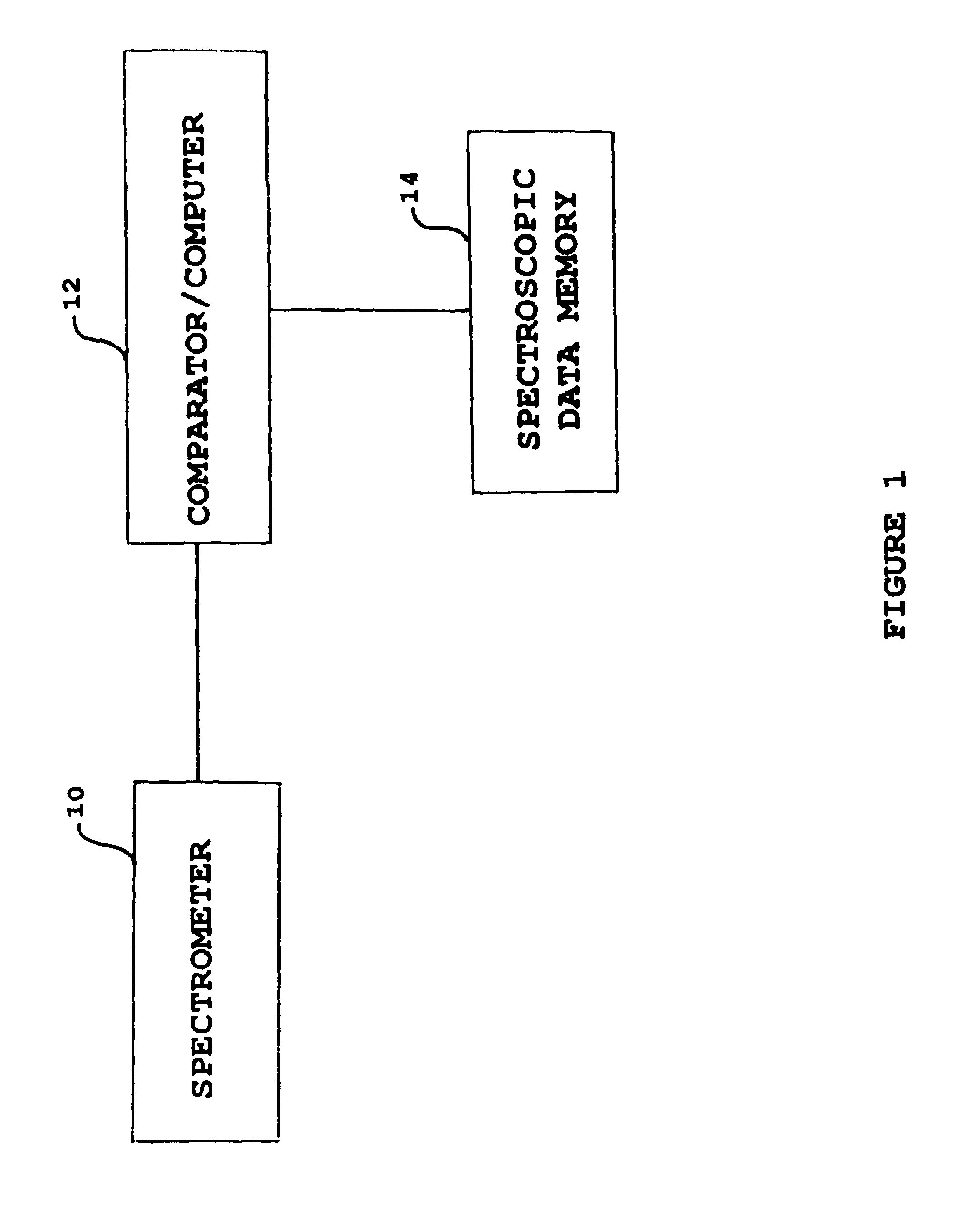
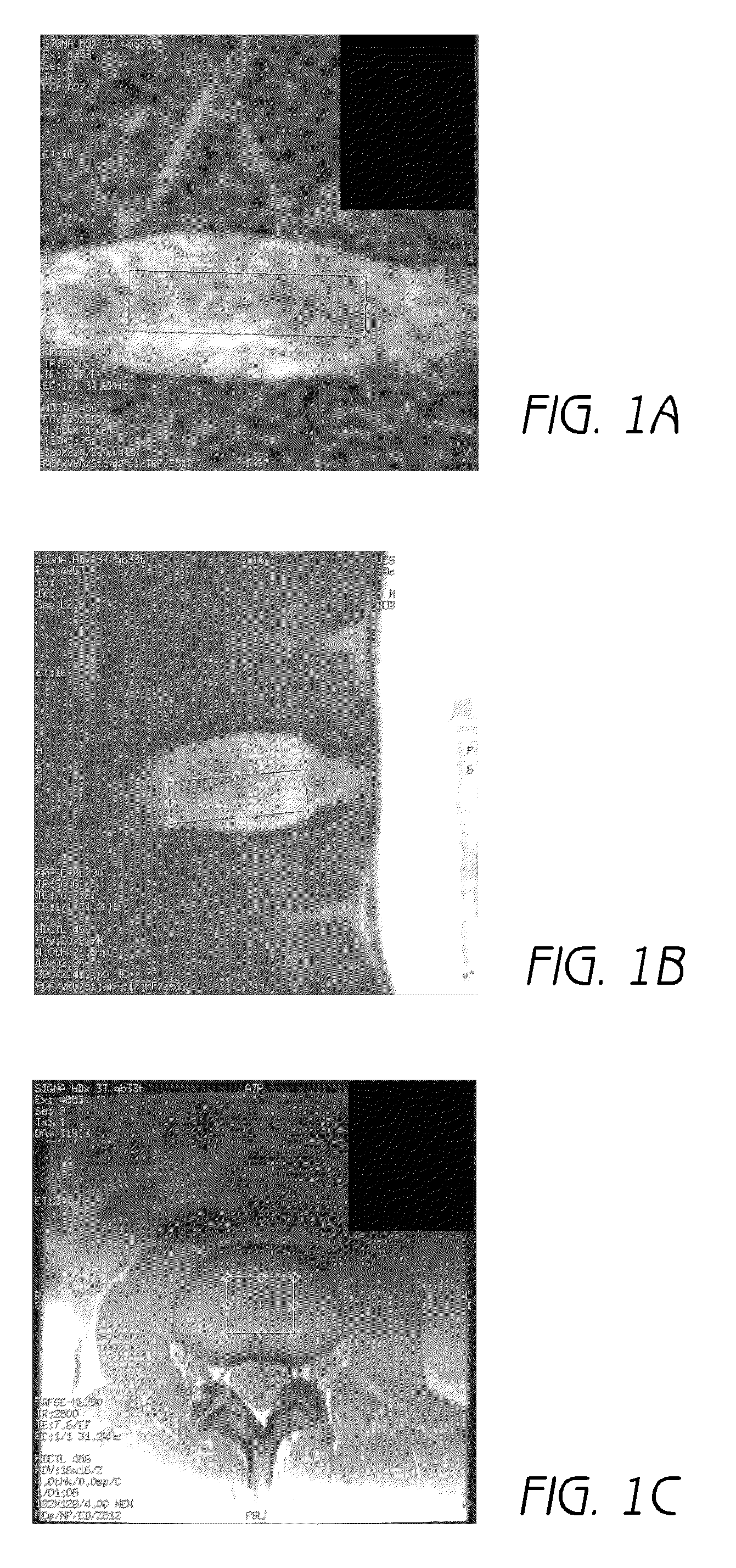
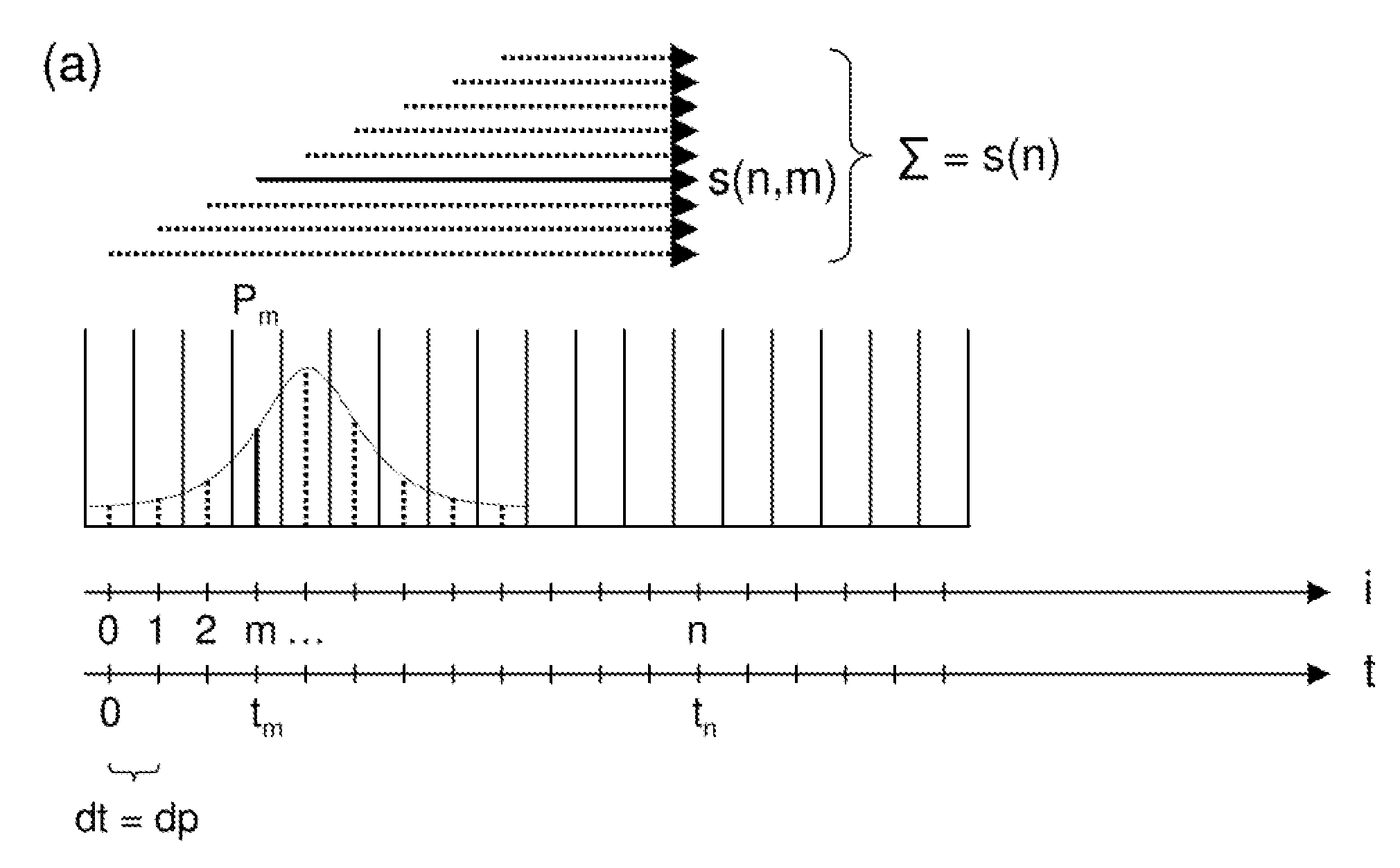Patents
Literature
181 results about "Magnetic resonance spectroscopic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
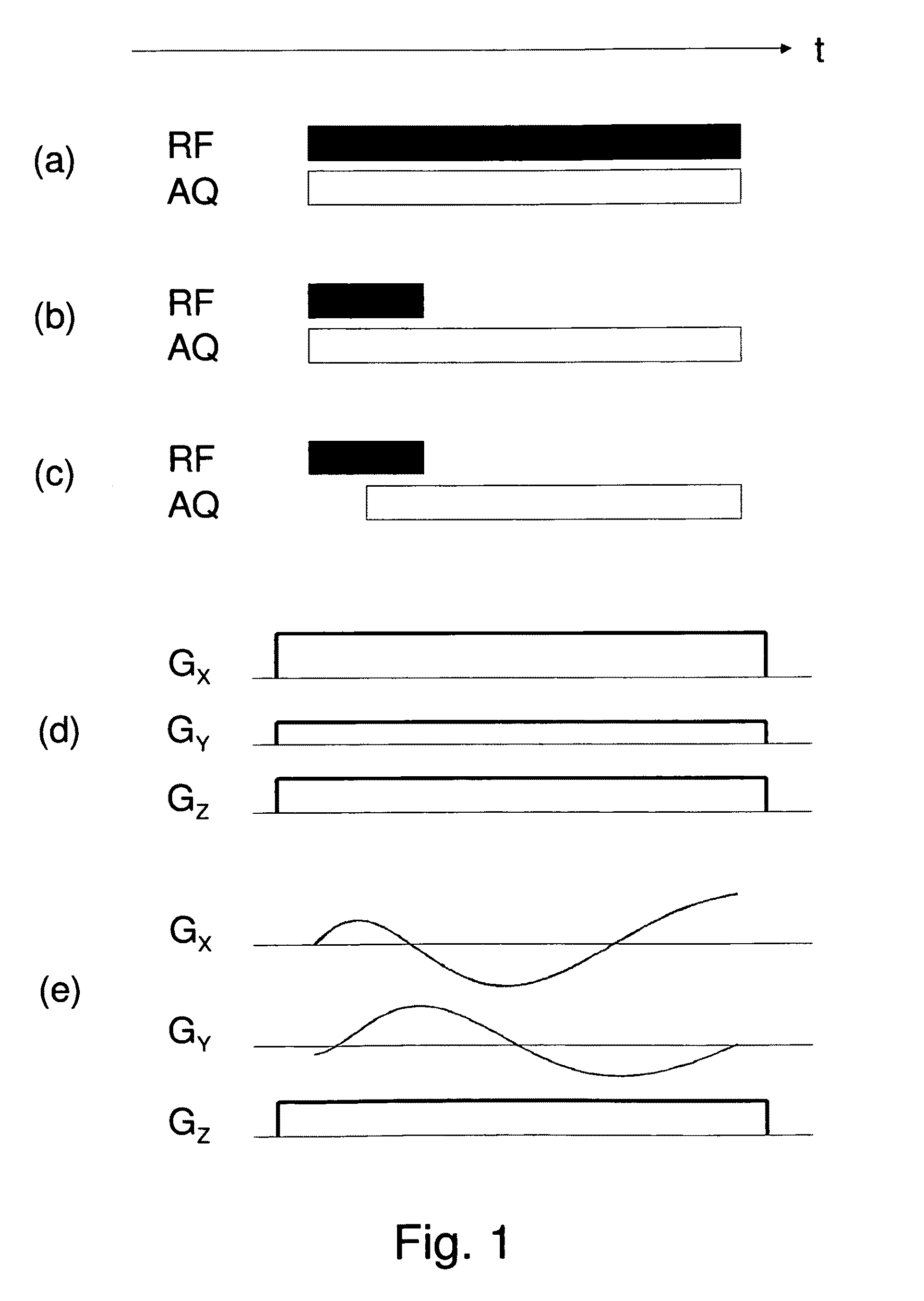
Localized two-dimensional shift correlated MR spectroscopy of human brain
InactiveUS7200430B2Measurements using NMR spectroscopyDiagnostic recording/measuringWhole bodySpectroscopy
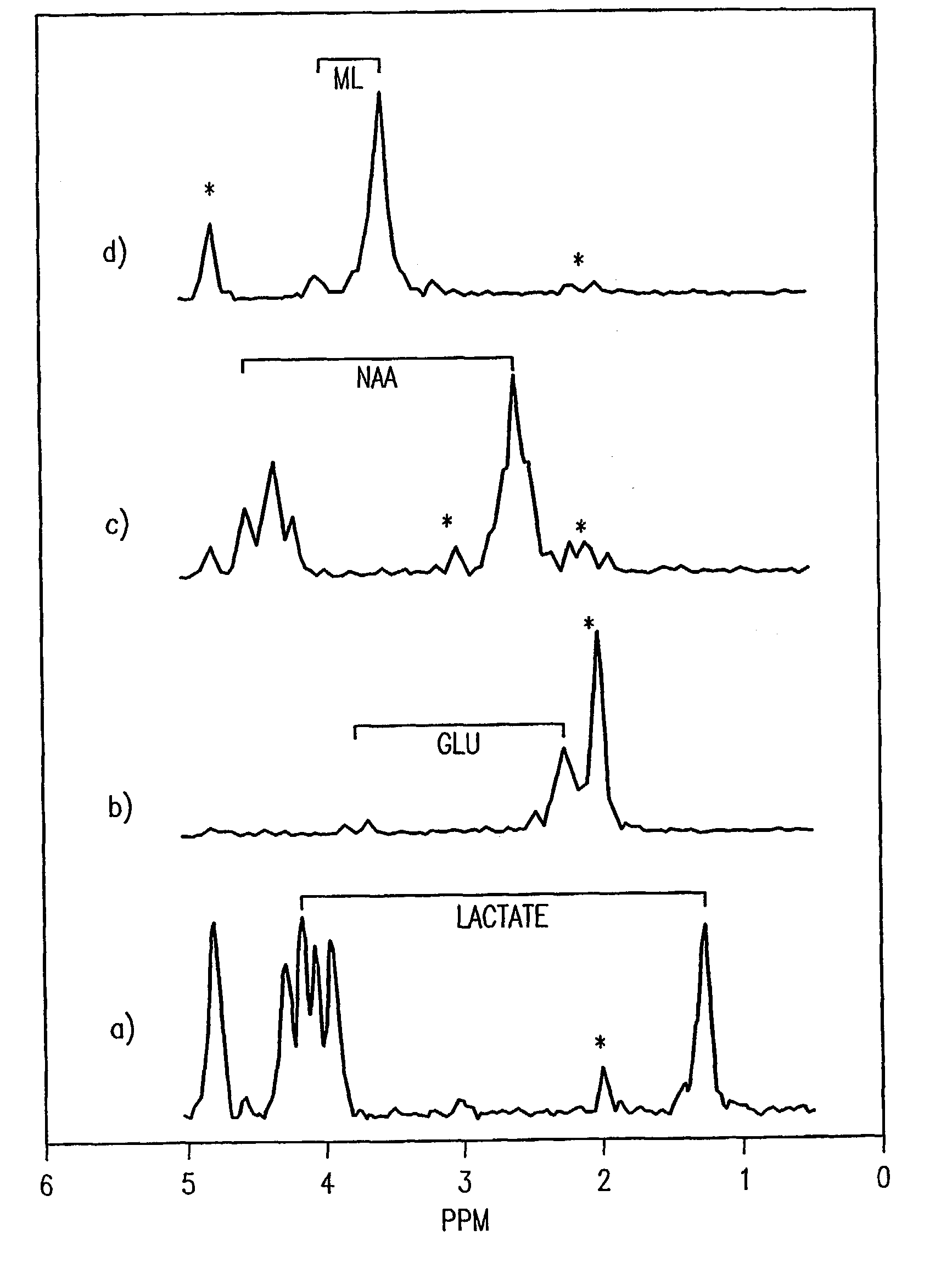
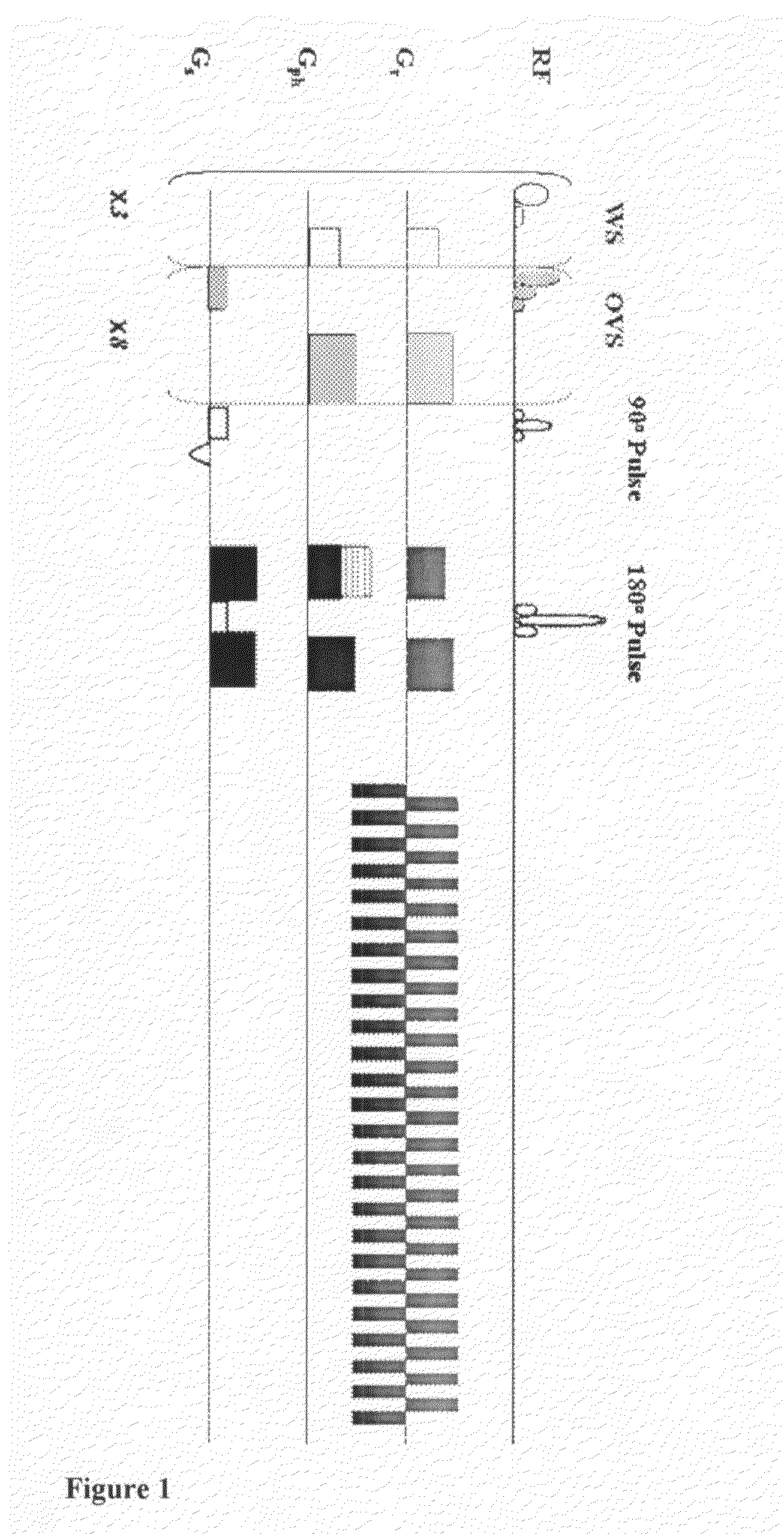
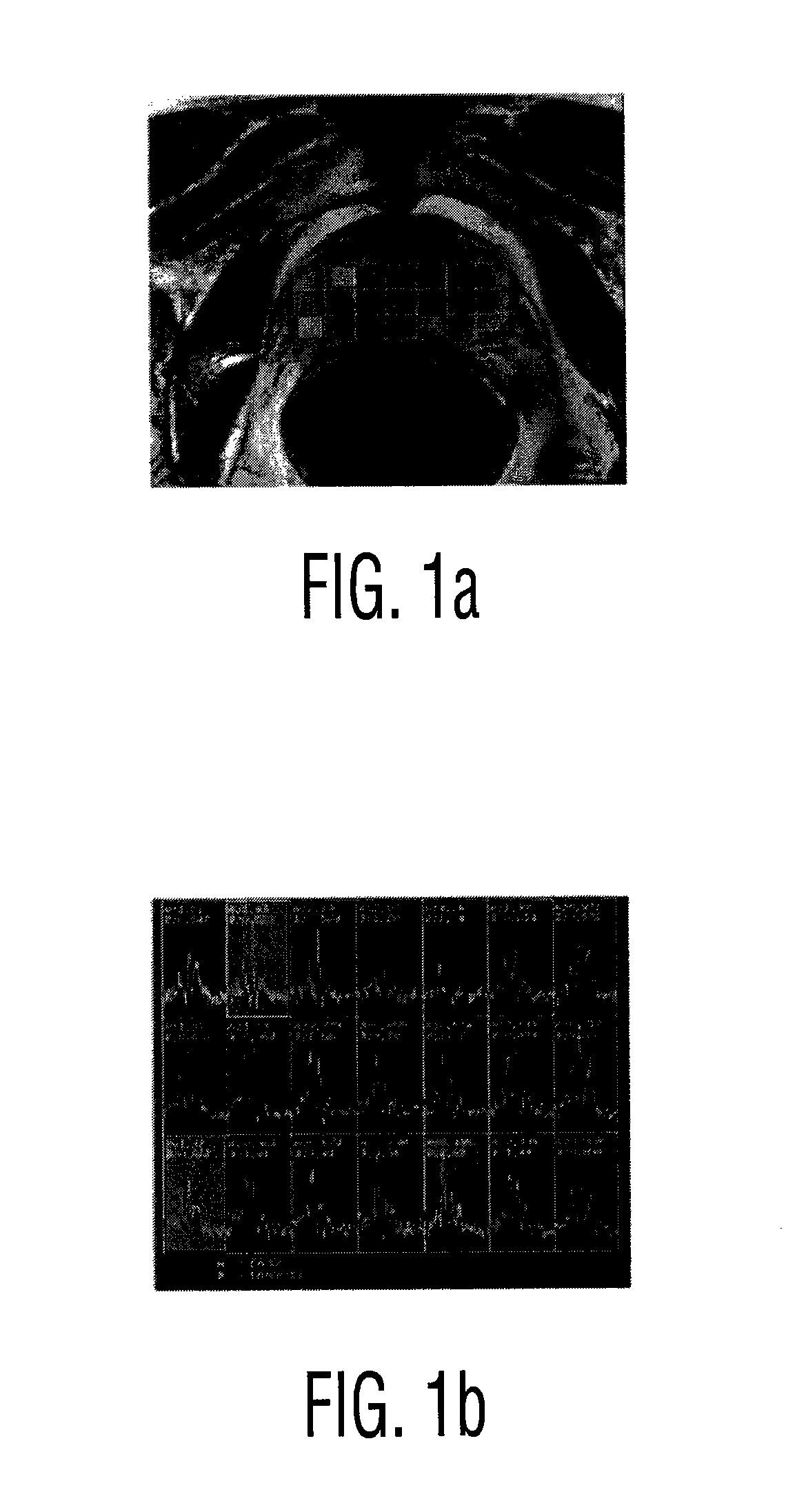
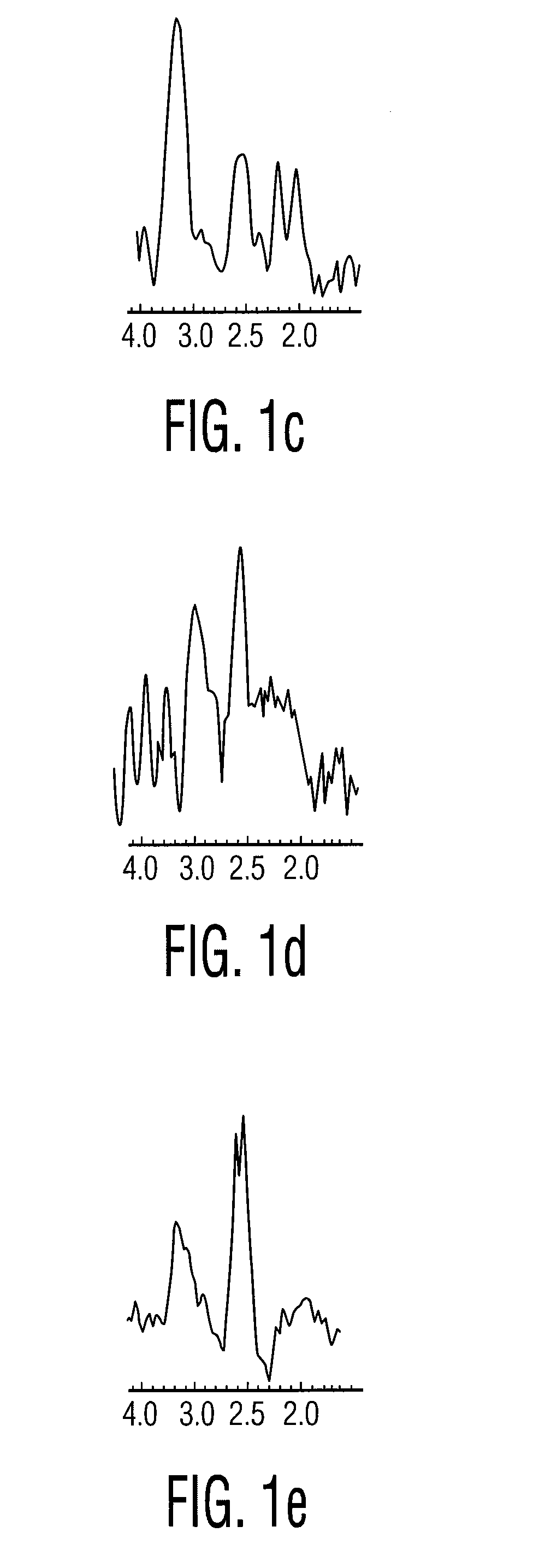
A two-dimensional (2D) chemical shift correlated MR spectroscopic (COSY) sequence integrated into a new volume localization technique (90°-180°-90°) for whole body MR Spectroscopy. Using the product operator formalism, a theoretical calculation of the volume localization as well as the coherence transfer efficiencies in 2D MRS is presented. A combination of different MRI transmit / receive rf coils is used. The cross peak intensities excited by the proposed 2D sequence are asymmetric with respect to the diagonal peaks. Localized COSY spectra of cerebral frontal and occipital gray / white matter regions in fifteen healthy controls are presented.
Owner:RGT UNIV OF CALIFORNIA
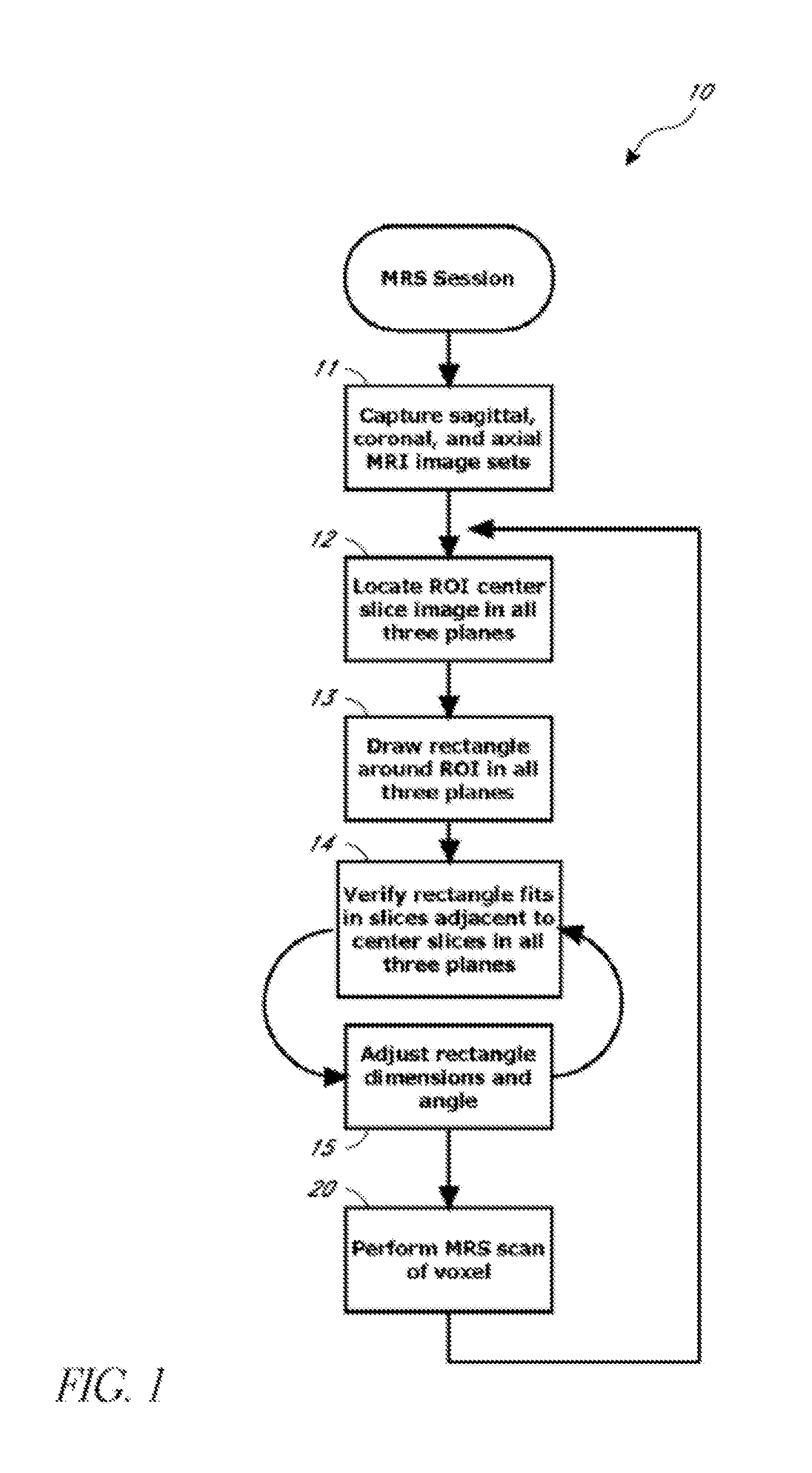
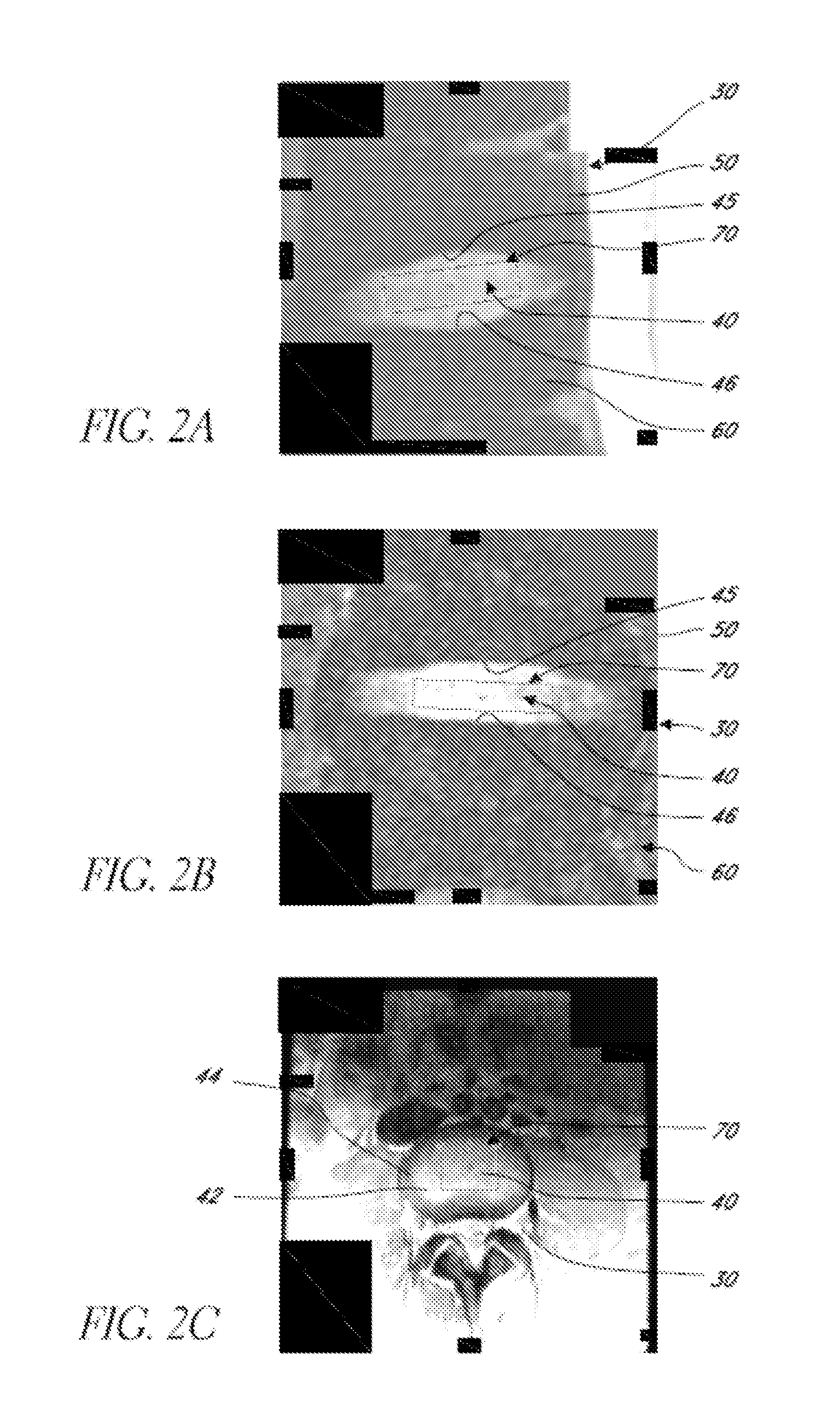
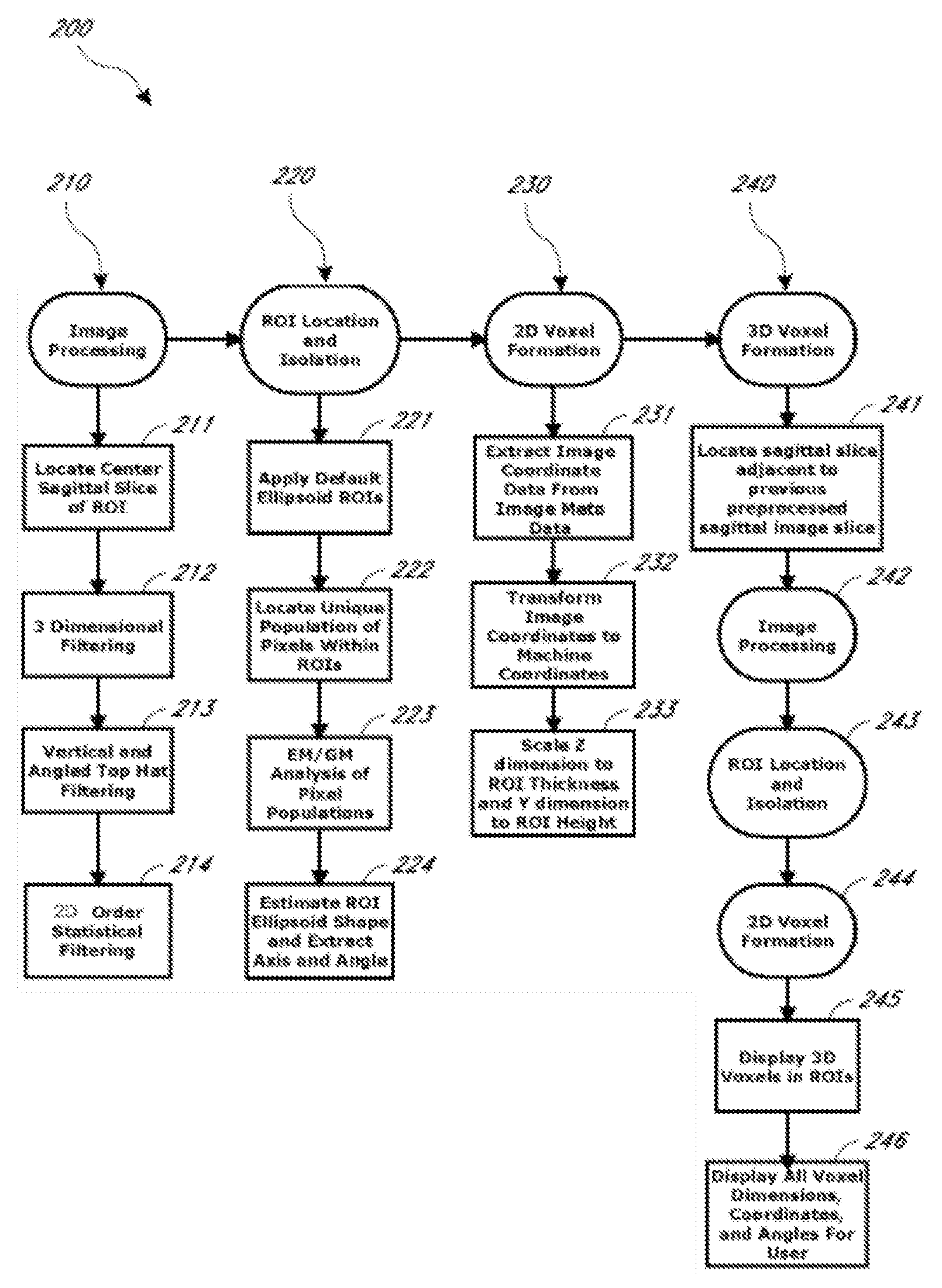
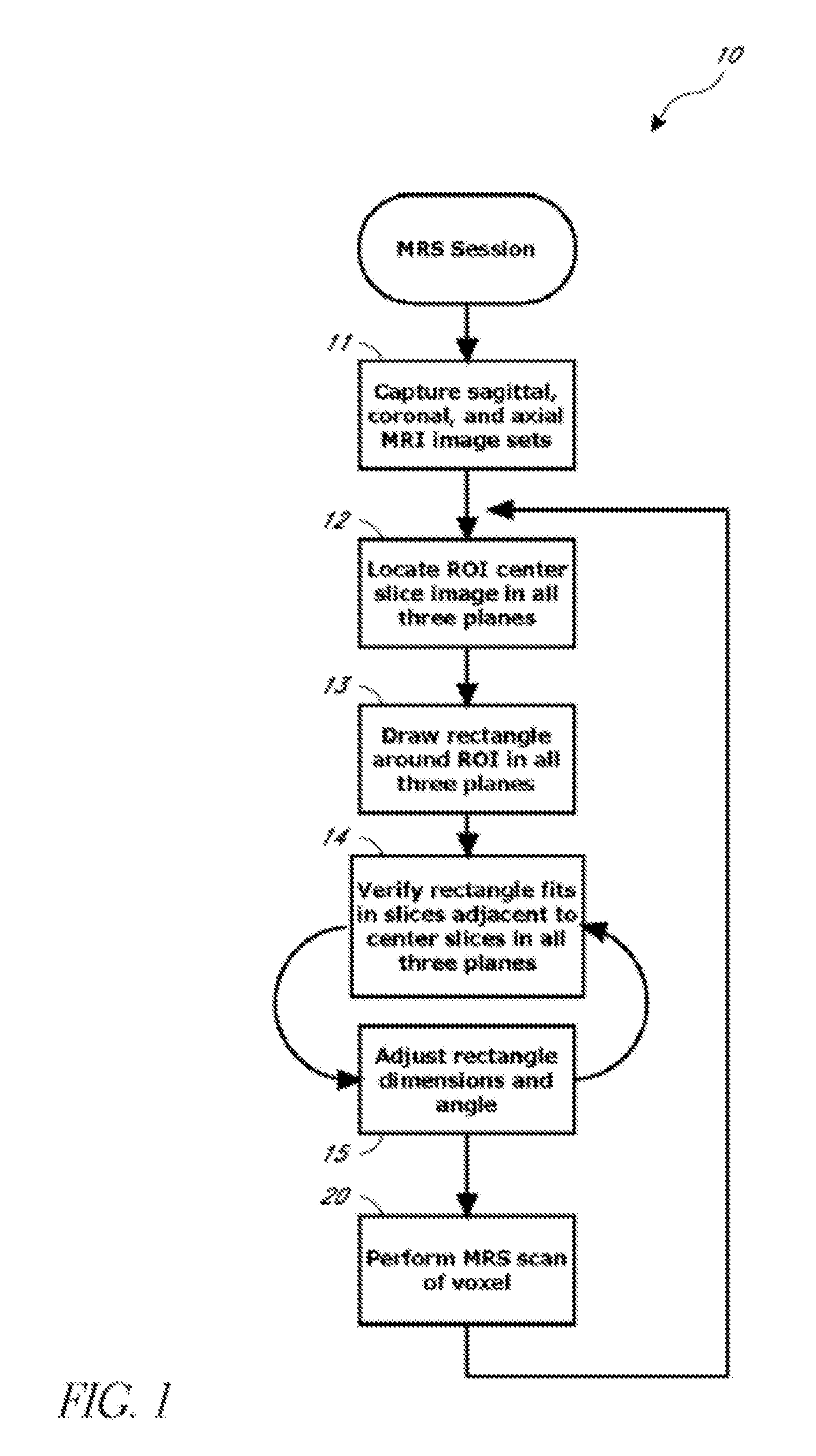
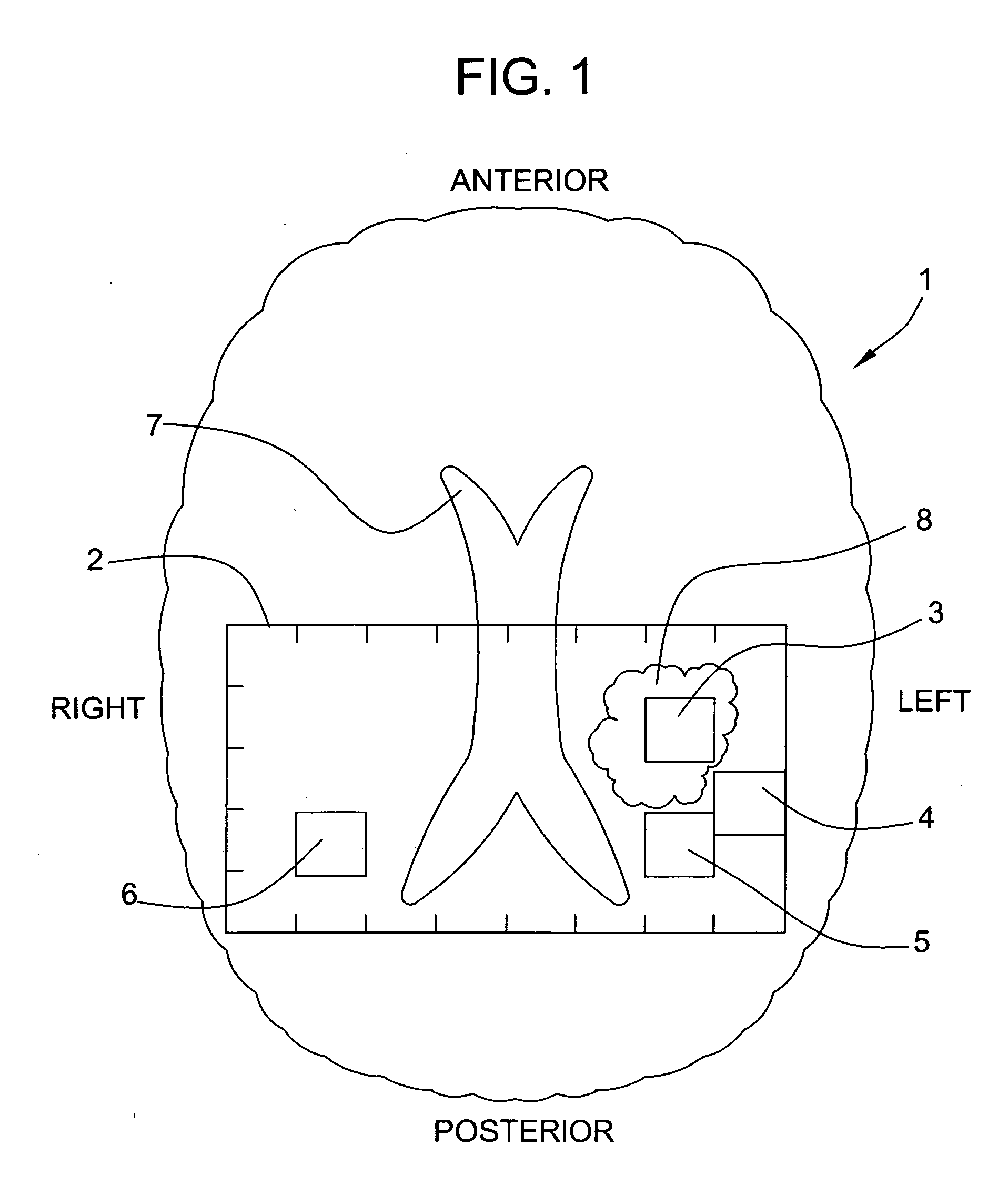
Systems and methods for automated voxelation of regions of interest for magnetic resonance spectroscopy
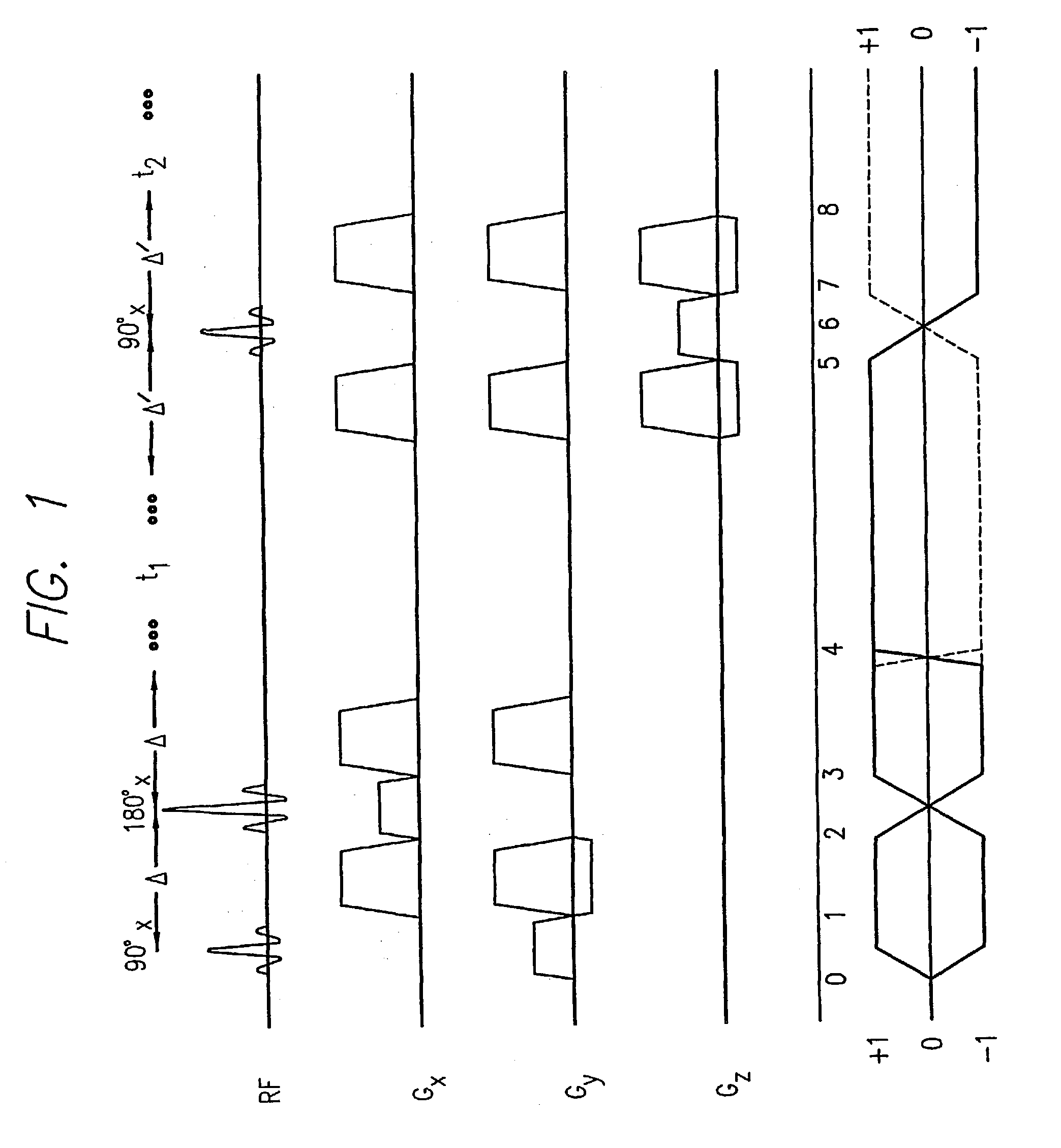
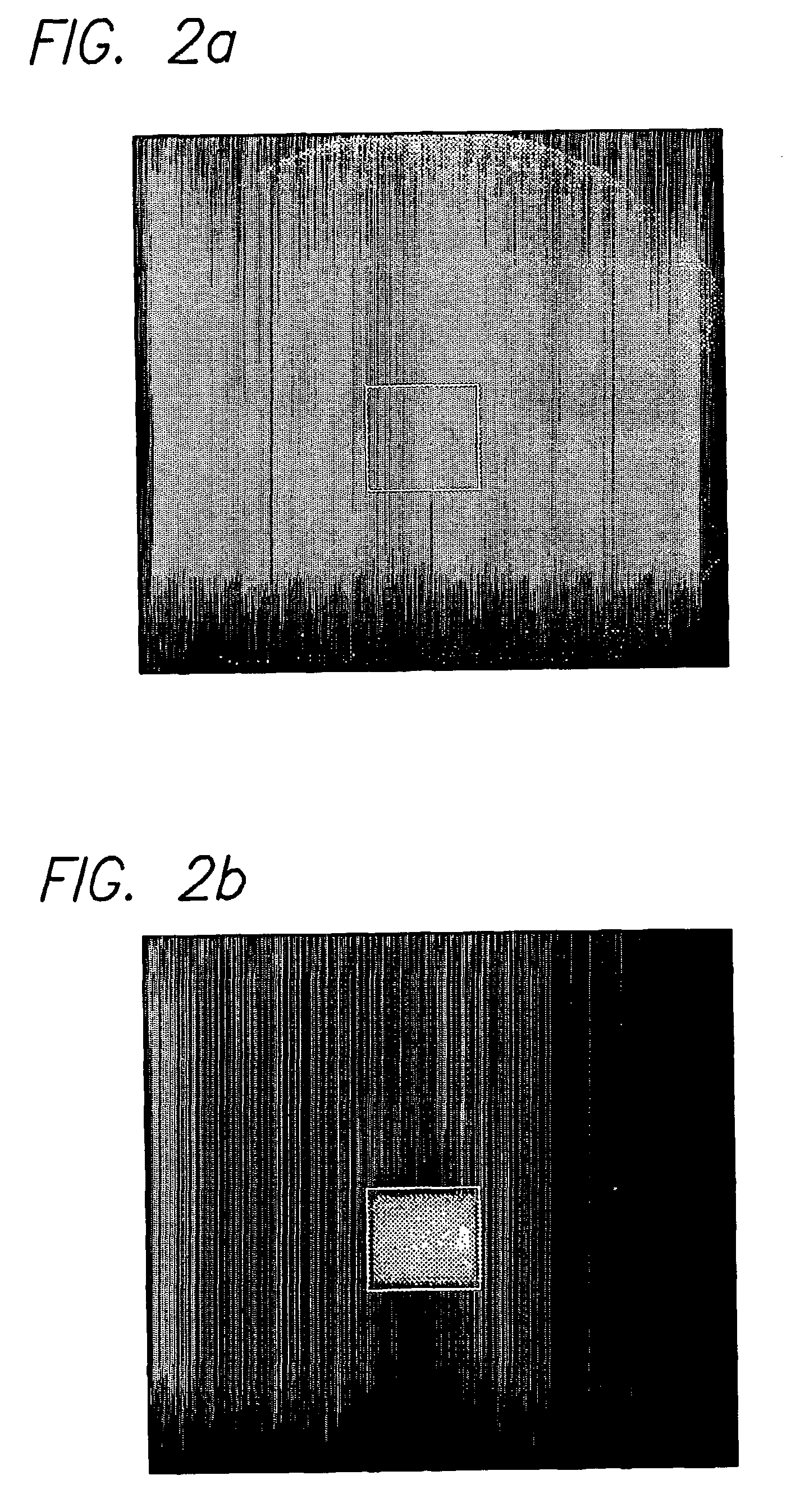
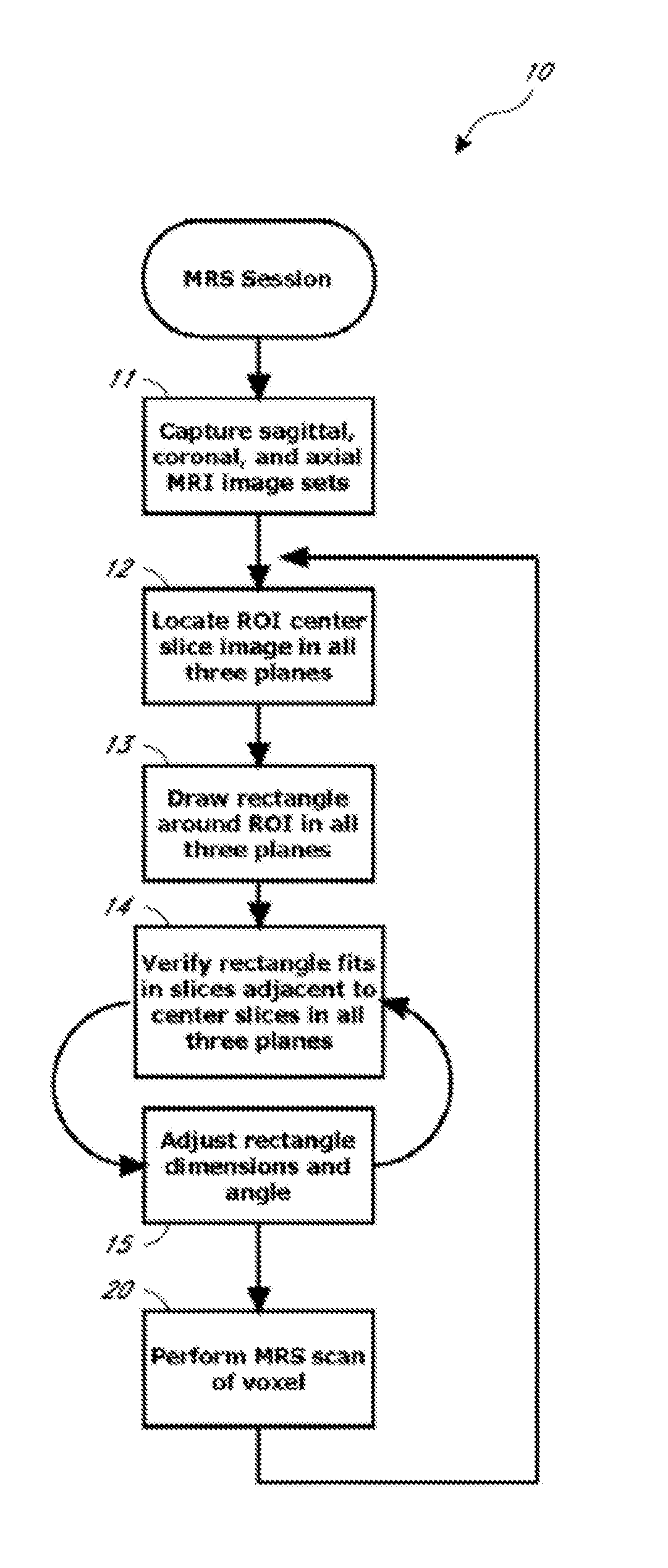
A system and method for automating an appropriate voxel prescription in a uniquely definable region of interest (ROI) in a tissue of a patient is provided, such as for purpose of conducting magnetic resonance spectroscopy (MRS) in the ROI. The dimensions and coordinates of a single three dimensional rectilinear volume (voxel) within a single region of interest (ROI) are automatically identified. This is done, in some embodiments by: (1) applying statistically identified ROI search areas within a field of view (FOV); (2) image processing an MRI image to smooth the background and enhance a particular structure useful to define the ROI; (3) identifying a population of pixels that define the particular structure; (4) performing a statistical analysis of the pixel population to fit a 2D model such as an ellipsoid to the population and subsequently fit a rectilinear shape within the model; (5) repetiting elements (1) through (4) using multiple images that encompass the 3D ROI to create a 3D rectilinear shape; (6) a repetition of elements (1) through (5) for multiple ROIs with a common FOV. A manual interface may also be provided, allowing for override to replace by manual prescription, assistance to identify structures (e.g. clicking on disc levels), or modifying the automated voxel (e.g. modify location, shape, or one or more dimensions).
Owner:ACLARION INC
Spectral resolution enhancement of magnetic resonance spectroscopic imaging
InactiveUS20100085050A1Spectral broadeningMeasurements using NMR imaging systemsElectric/magnetic detectionTime domainMagnetic resonance spectroscopic
A method and apparatus for enhancing the spectral resolution of magnetic resonance spectroscopic (MRS) measurements include receiving time domain echo data from an MRS measurement for an MRS volume in a subject. Also received are high spatial resolution complex signal values within the MRS volume based on magnetic resonance imaging (MRI) measurements. Frequency-domain content is determined for the echo data based at least in part on the complex signal values. For example, in some embodiments, receiving complex signal values includes receiving high spatial resolution complex signal values within the MRS volume for each of two different echo time settings. The frequency-domain content of the echo data is corrected for a lineshape profile based on high resolution frequency dispersion values for the MRS volume determined from differences in the complex signal values for the two different echo time settings.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
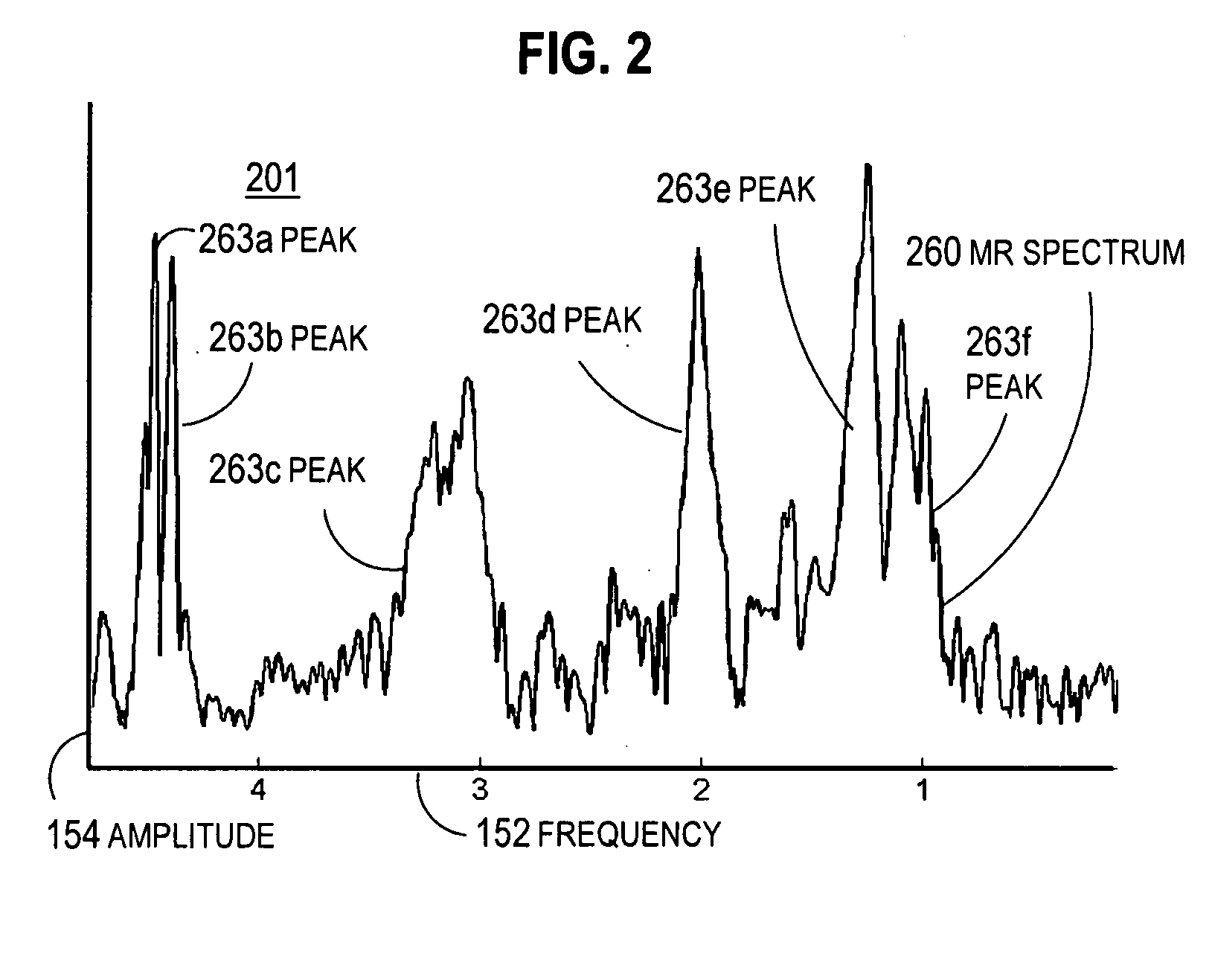
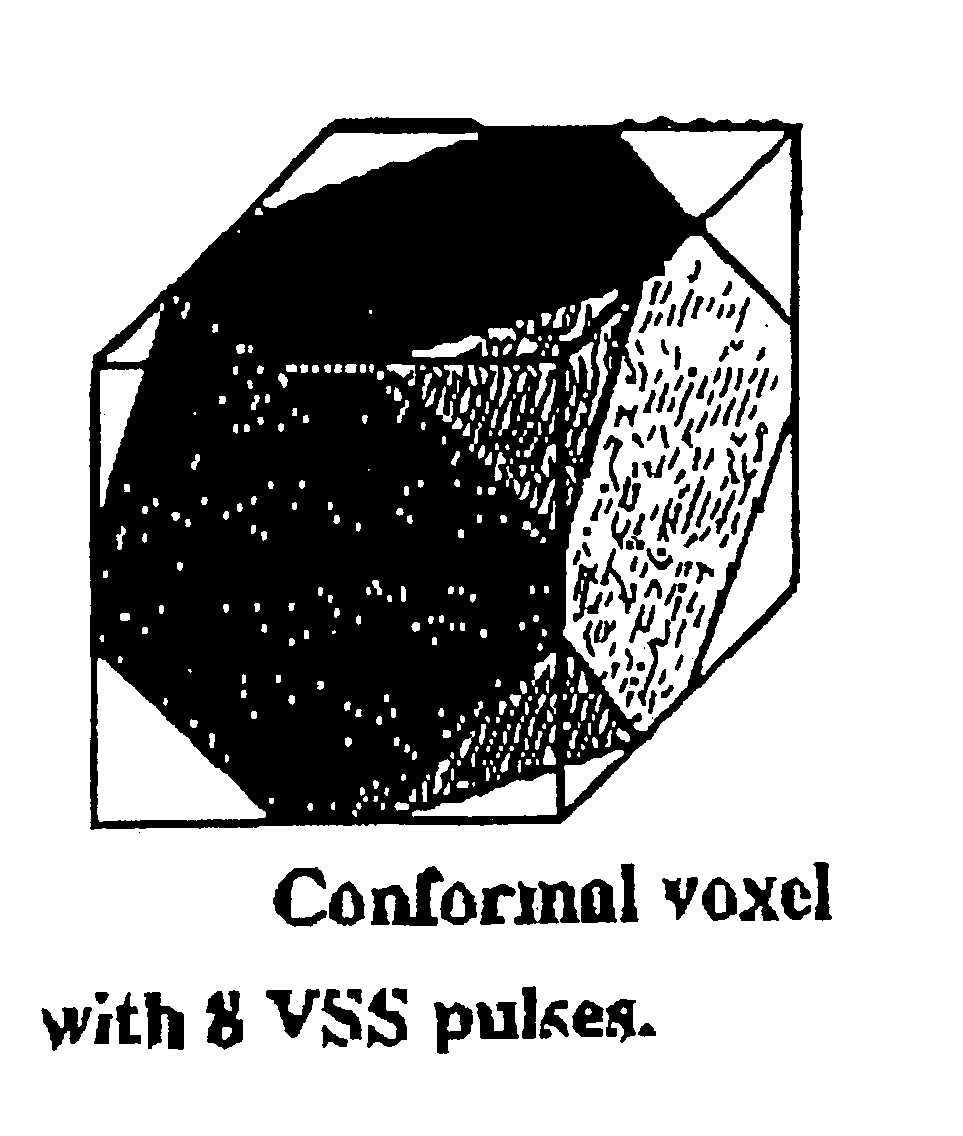
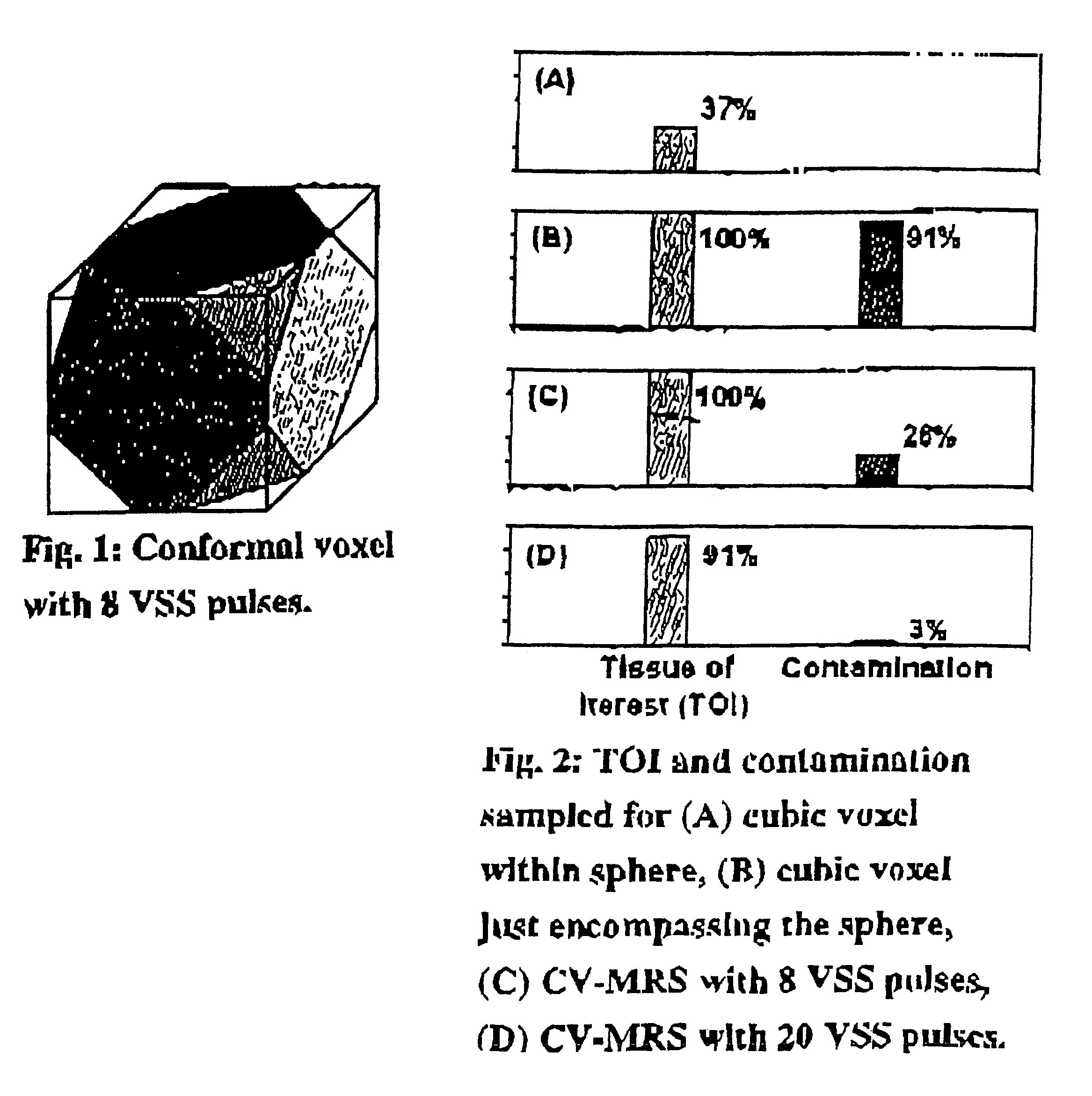
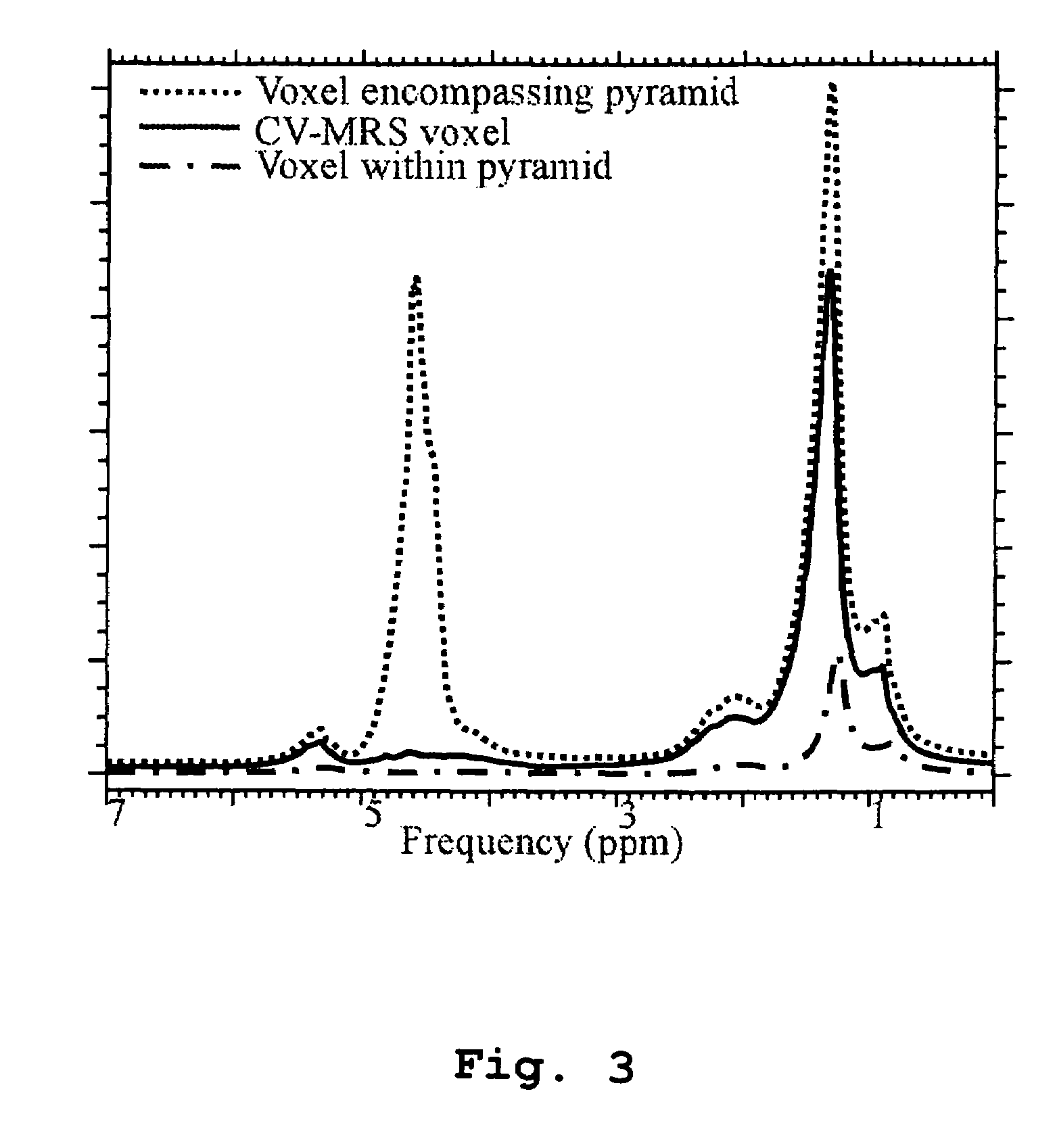
Magnetic resonance spectroscopy using a conformal voxel
InactiveUS7319784B2Improving prescriptionAdd featureMagnetic measurementsCharacter and pattern recognitionVoxelSelective excitation
The goal was to automate and optimize the shaping and positioning of a shape-specific / conformal voxel that conforms to any volume of interest, such as a cranial lesion, to allow conformal voxel magnetic resonance spectroscopy (CV-MRS). We achieved this by using a computer program that optimizes the shape, size, and location of a convex polyhedron within the volume of interest. The sides of the convex polyhedron are used to automatically prescribe the size and location of selective excitation voxels and / or spatial saturation slices. For a spherically-shaped, phantom-simulated lesion, CV-MRS increased the signal from the lesion by a factor of 2.5 compared to a voxel completely inside the lesion. CV-MRS reduces the voxel prescription time, operator subjectivity, and acquisition time.
Owner:NAT RES COUNCIL OF CANADA
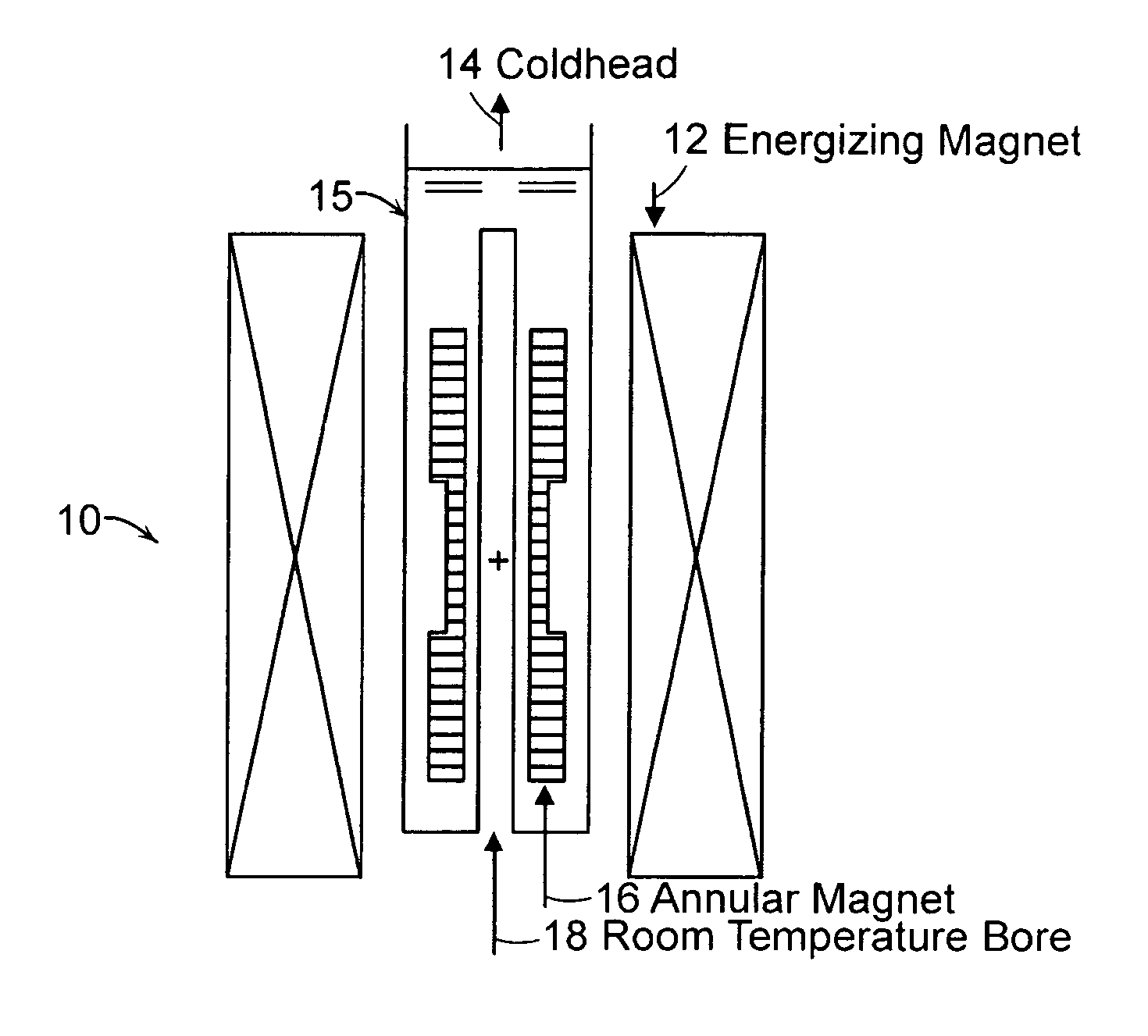
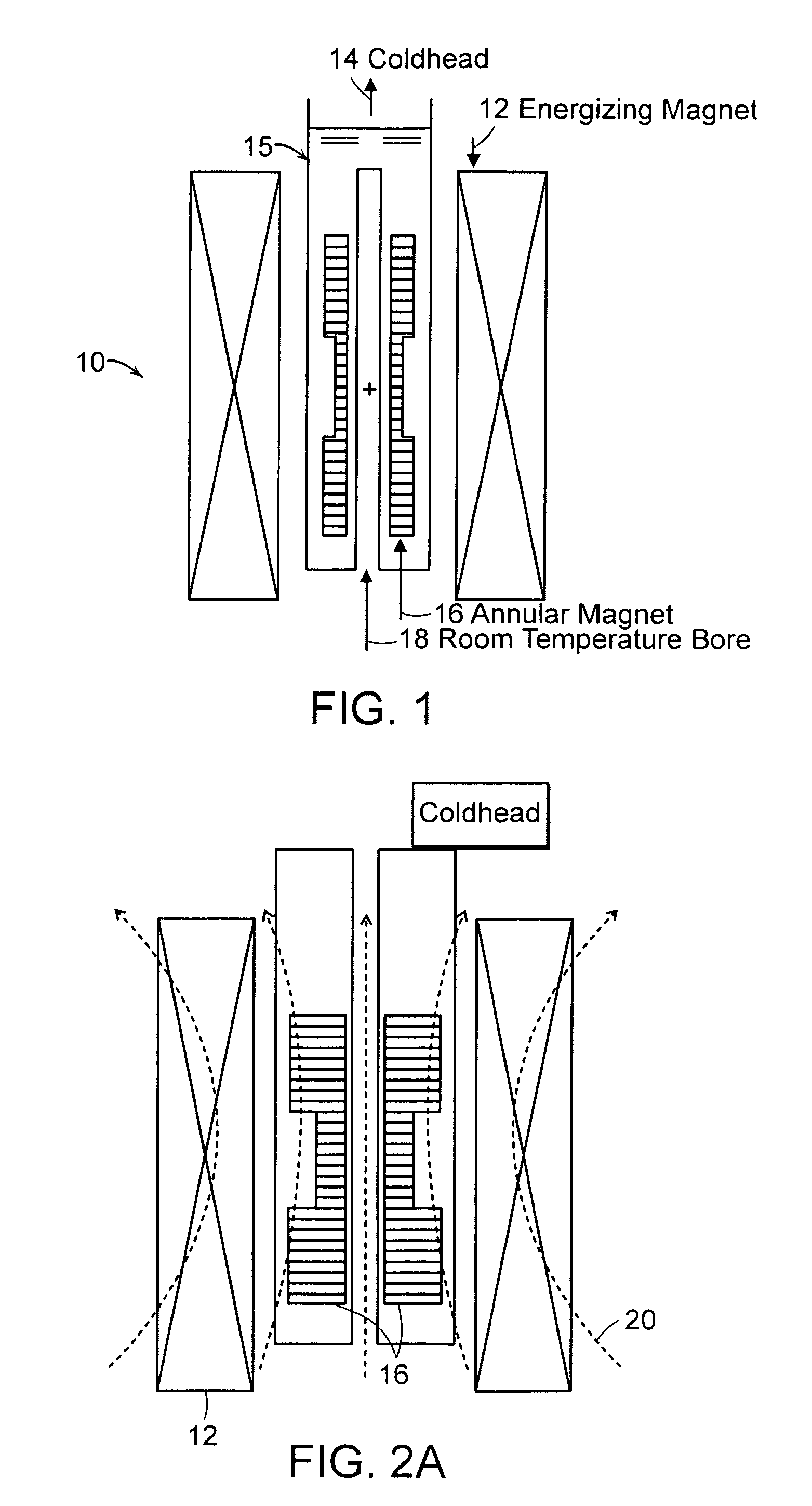
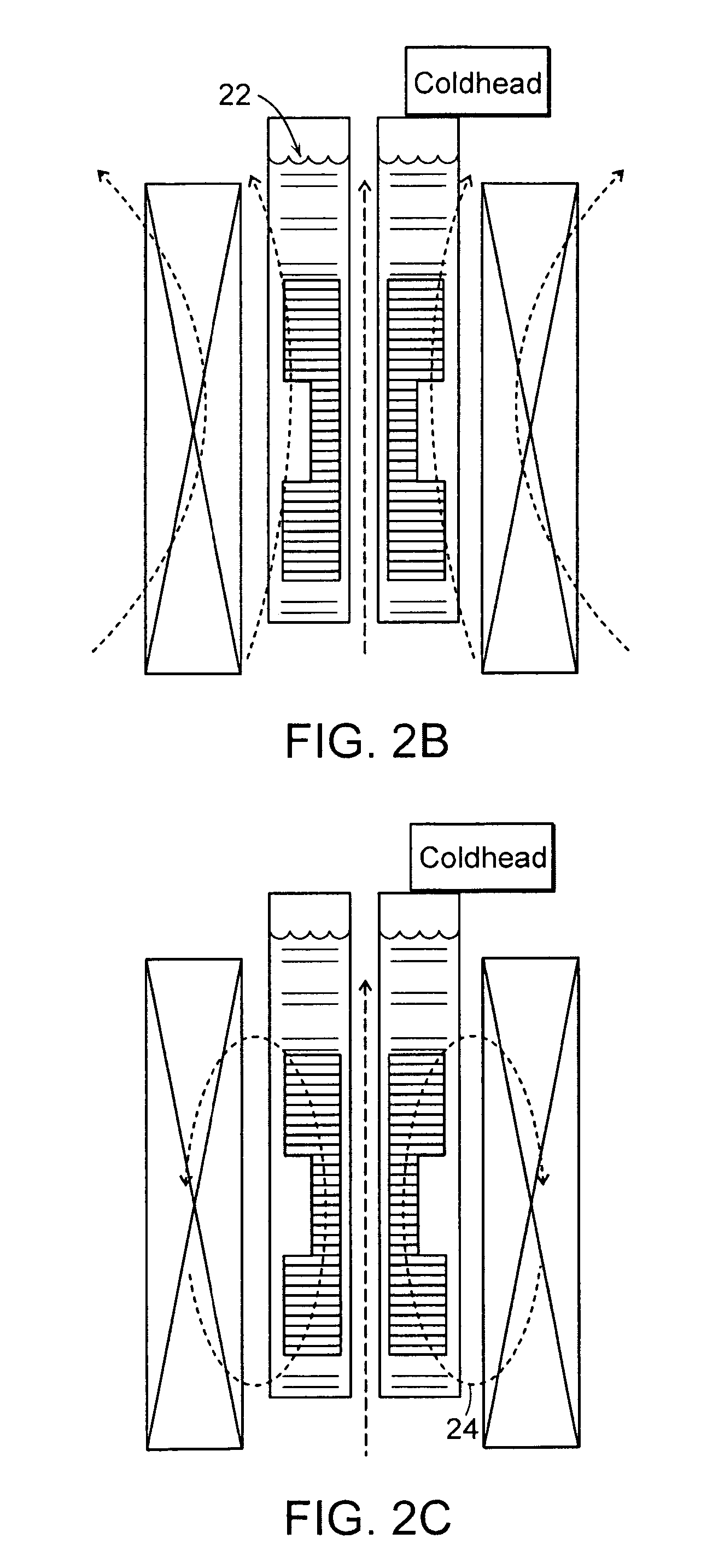
Annular magnet system for magnetic resonance spectroscopy
Owner:MASSACHUSETTS INST OF TECH +1
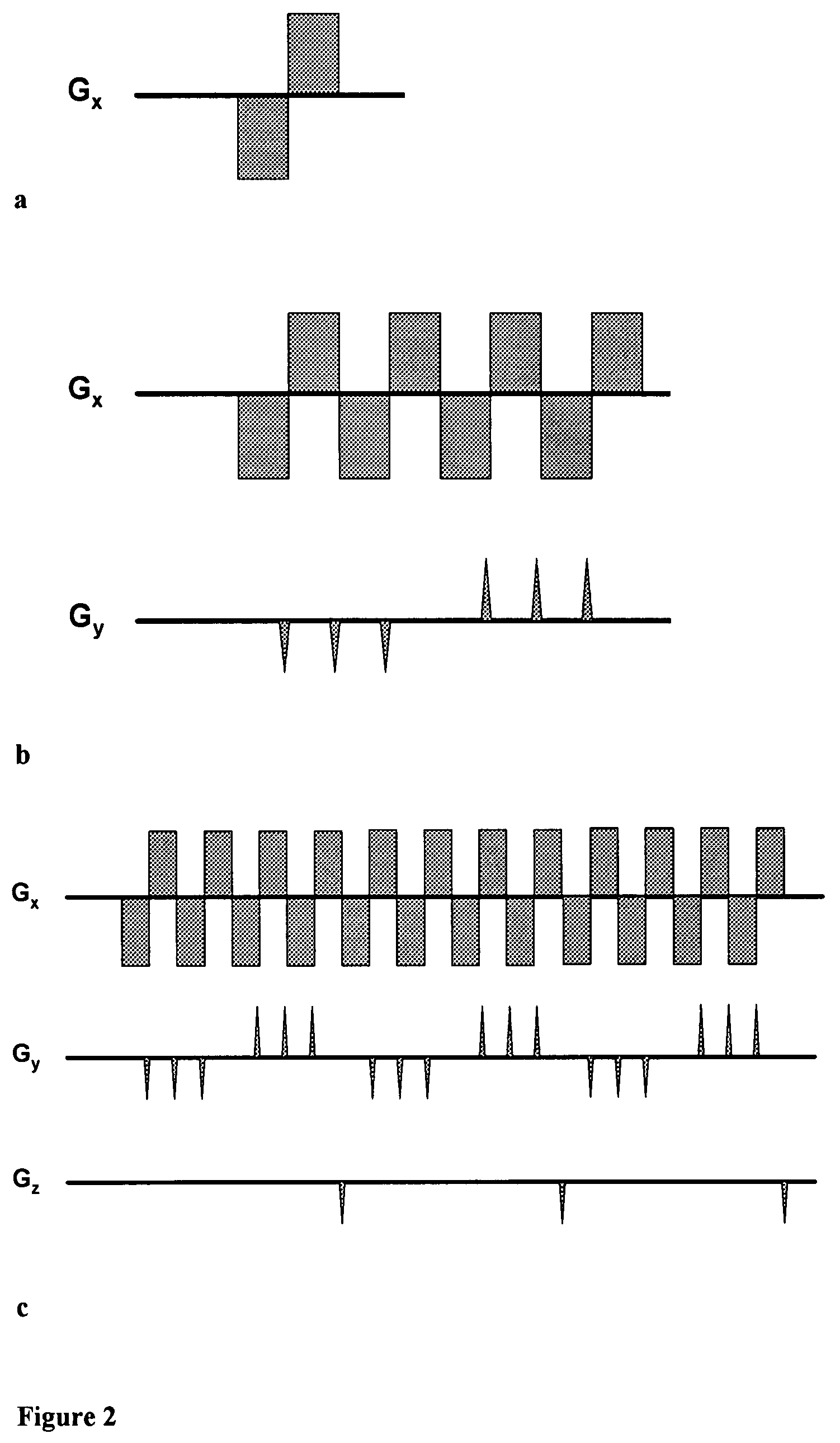
Magnetic resonance spectroscopy with sparse spectral sampling and interleaved dynamic shimming
InactiveUS7683614B2Maximize efficiencyMaximize sensitivityMagnetic measurementsElectric/magnetic detectionMagnetic field gradientSpectral dimension
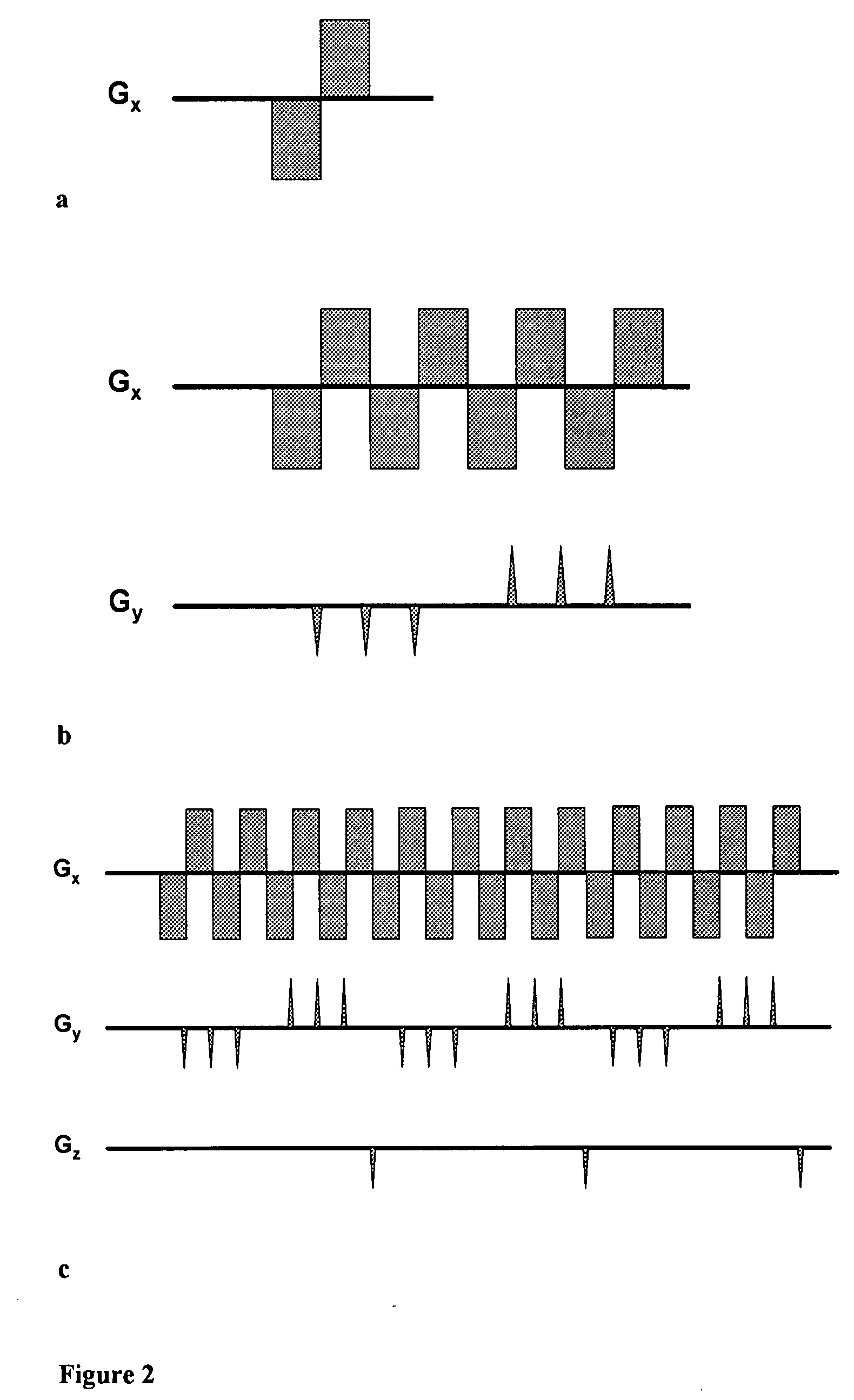
The present invention relates to a magnetic resonance spectroscopic imaging (MRSI) method, specifically to a magnetic resonance spectroscopic imaging method with up to three spatial dimensions and one spectral dimension. Interleaving dynamically switched magnetic field gradients into the spectroscopic encoding scheme enables multi-region shimming in a single shot to compensate the spatially varying spectral line broadening resulting from local magnetic field gradients. The method also employs sparse spectral sampling with controlled spectral aliasing and nonlinear sampling density to maximize encoding speed, data sampling efficiency and sensitivity.
Owner:POSSE STEFAN
Magnetic resonance spectroscopy with sparse spectral sampling and interleaved dynamic shimming
InactiveUS20070252597A1Maximize encoding speedMaximize data sampling efficiencyMagnetic measurementsElectric/magnetic detectionMagnetic field gradientSpectral dimension
The present invention relates to a magnetic resonance spectroscopic imaging (MRSI) method, specifically to a magnetic resonance spectroscopic imaging method with up to three spatial dimensions and one spectral dimension. Interleaving dynamically switched magnetic field gradients into the spectroscopic encoding scheme enables multi-region shimming in a single shot to compensate the spatially varying spectral line broadening resulting from local magnetic field gradients. The method also employs sparse spectral sampling with controlled spectral aliasing and nonlinear sampling density to maximize encoding speed, data sampling efficiency and sensitivity.
Owner:POSSE STEFAN
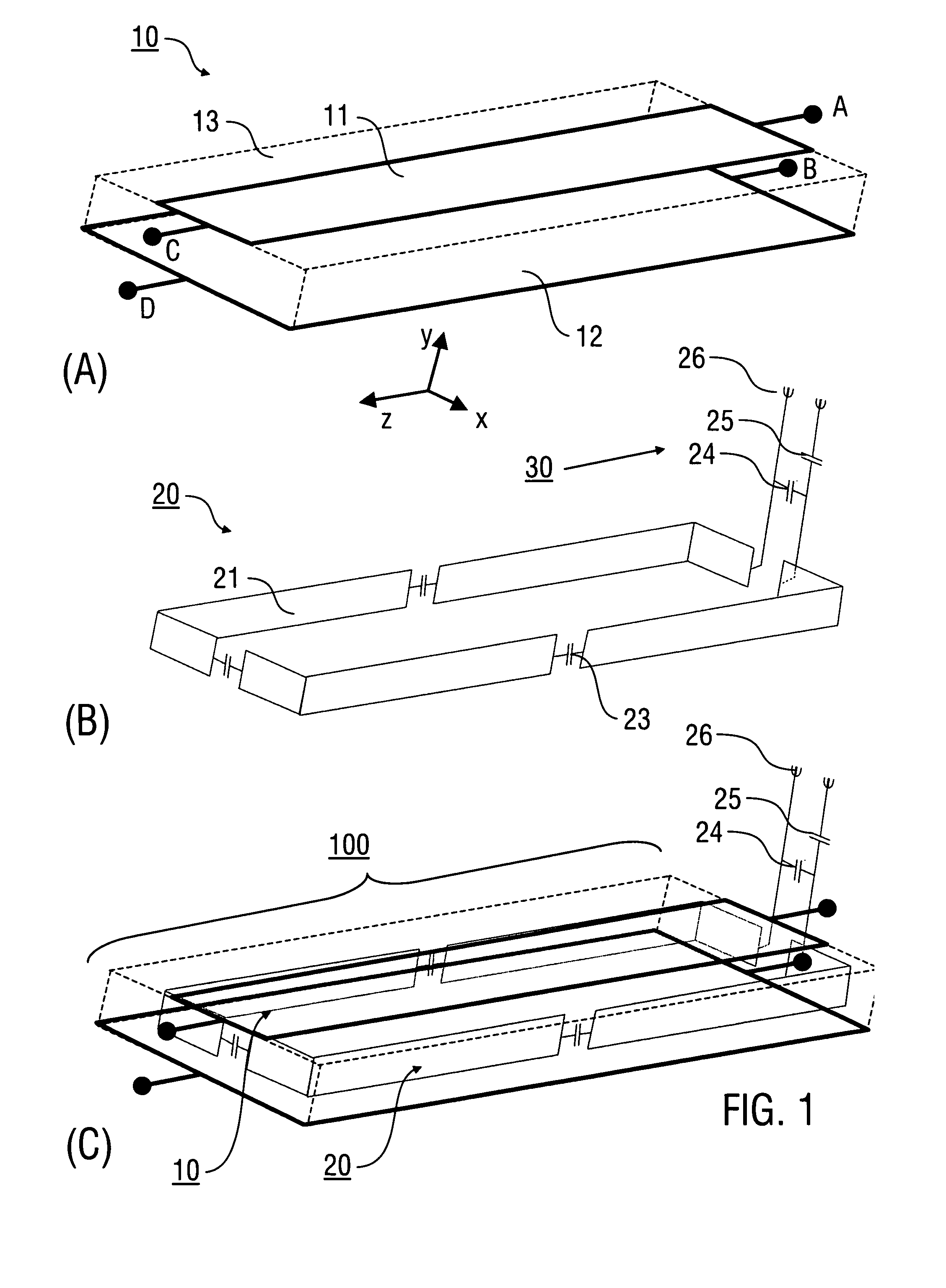
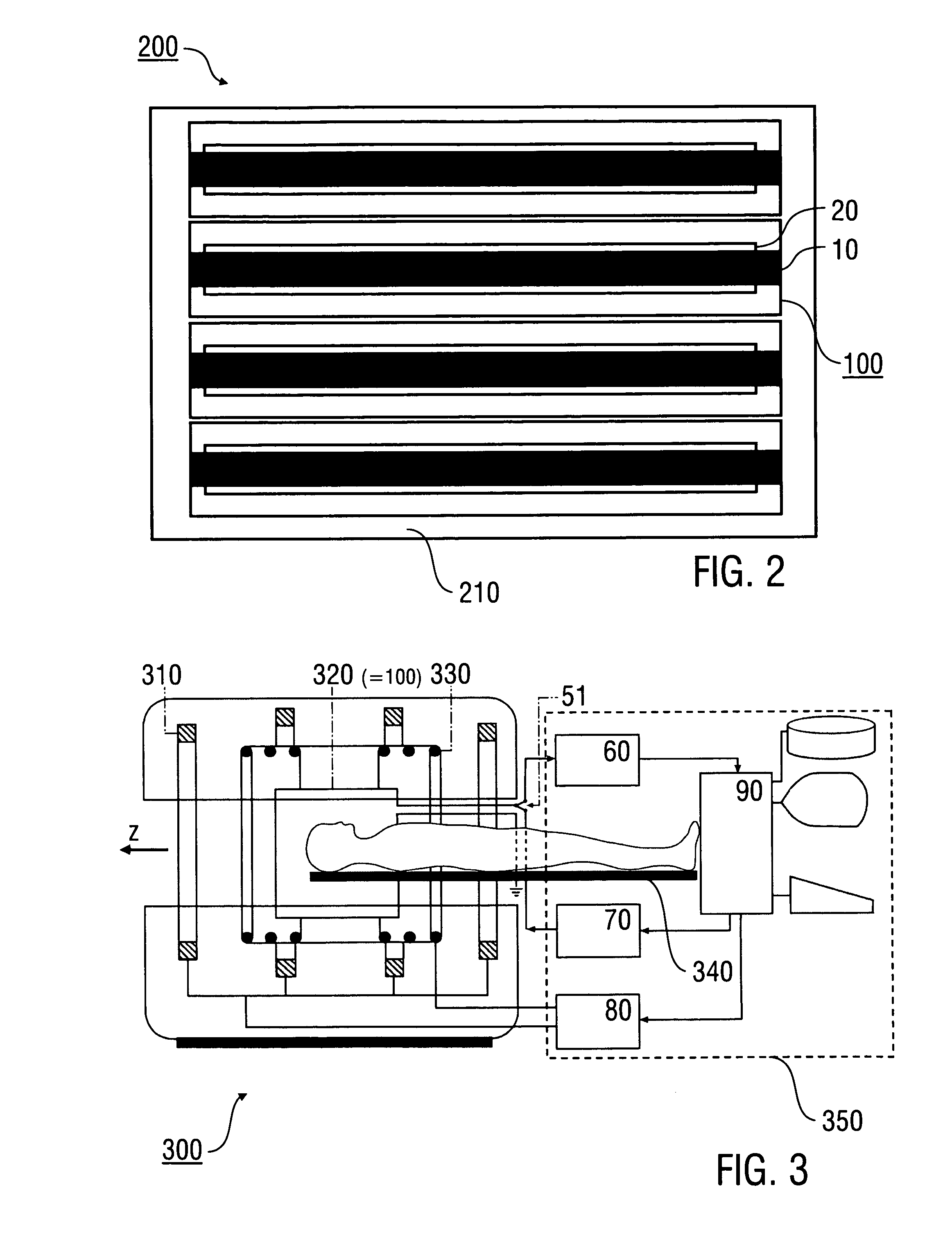
Stripline antenna and antenna array for a magnetic resonance device
InactiveUS20100213941A1Antenna structure is simpleSimple structureElectric/magnetic detectionMeasurements using magnetic resonanceMagnetic resonance spectroscopicStripline resonator
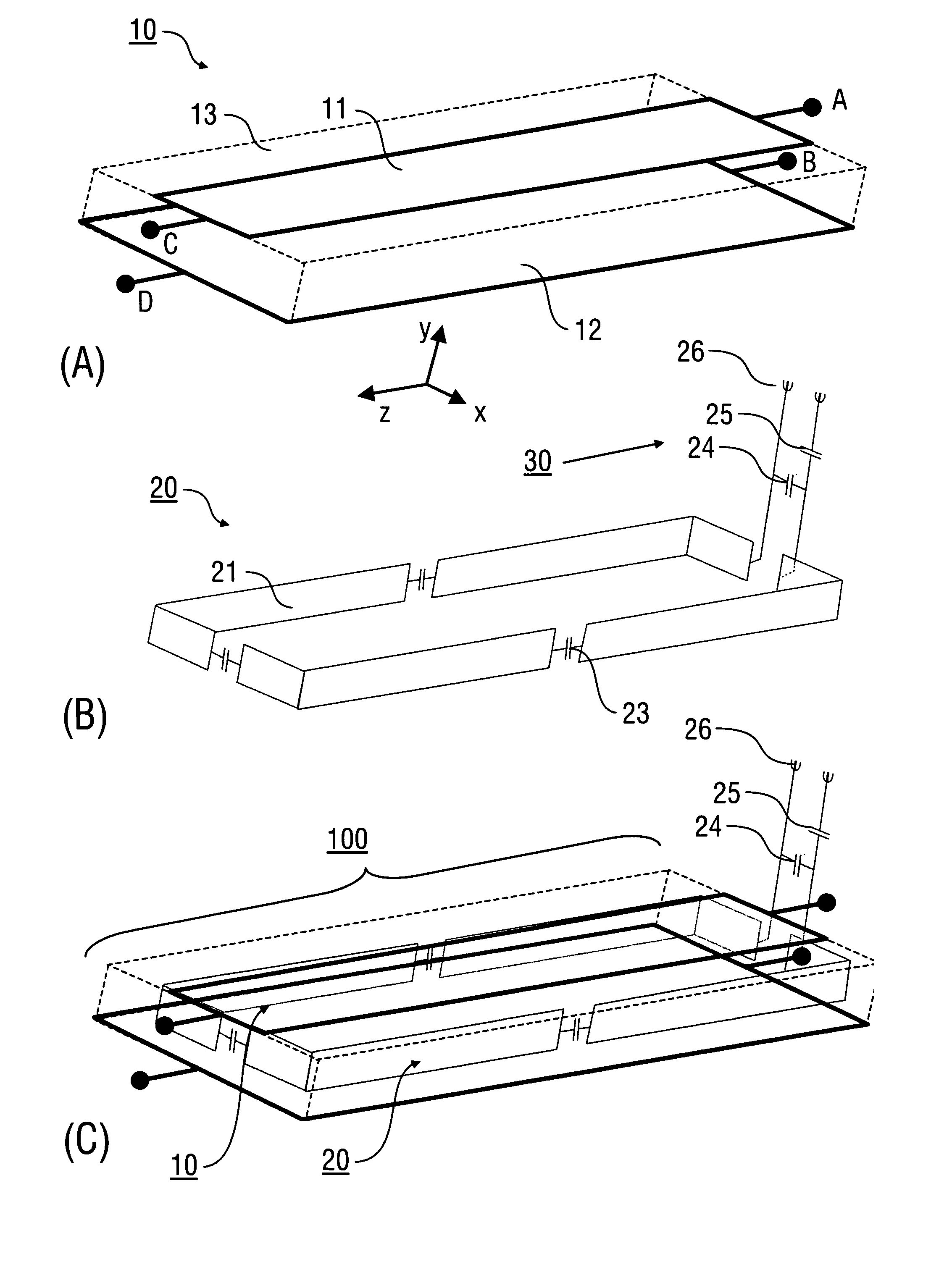
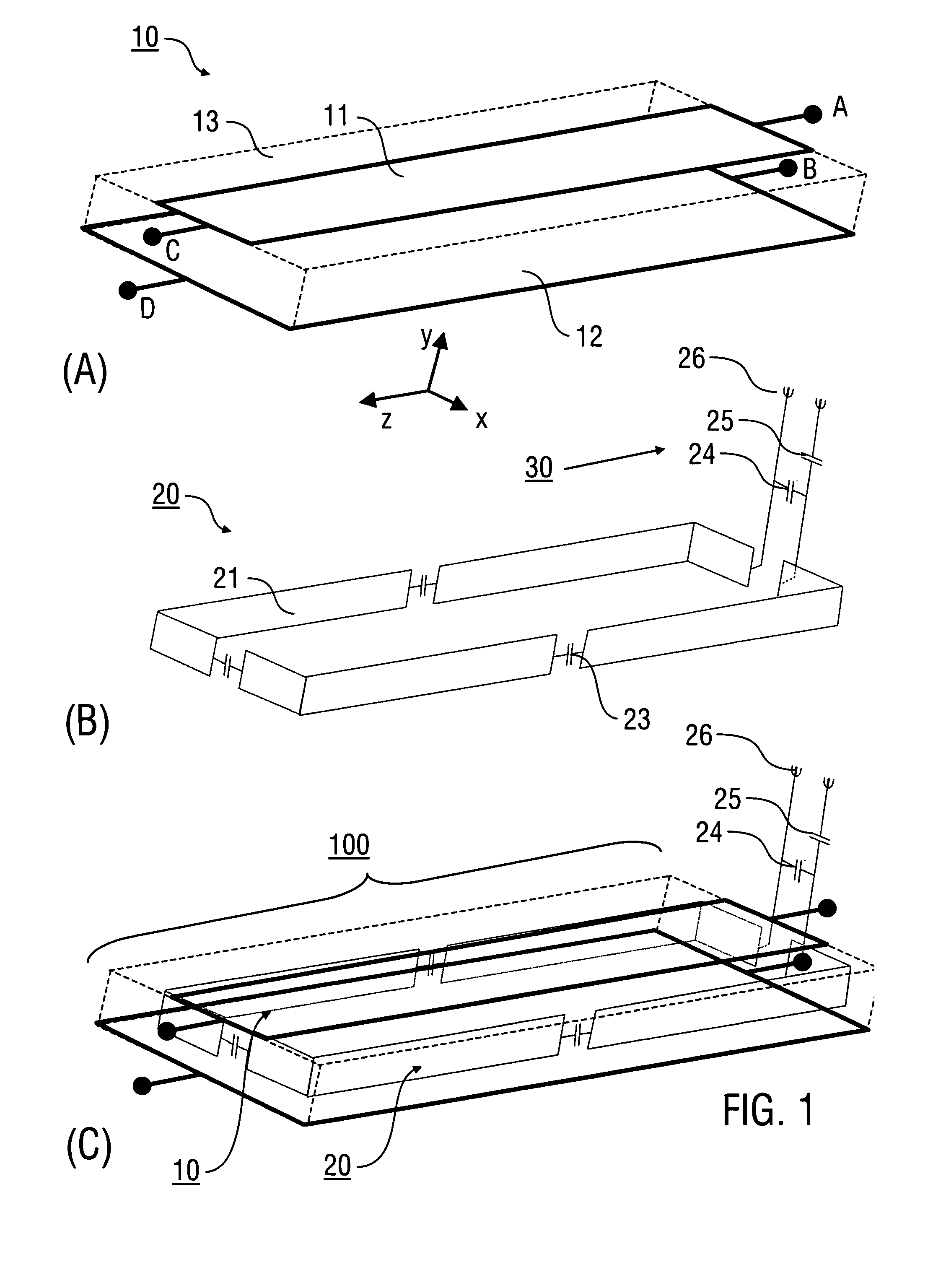
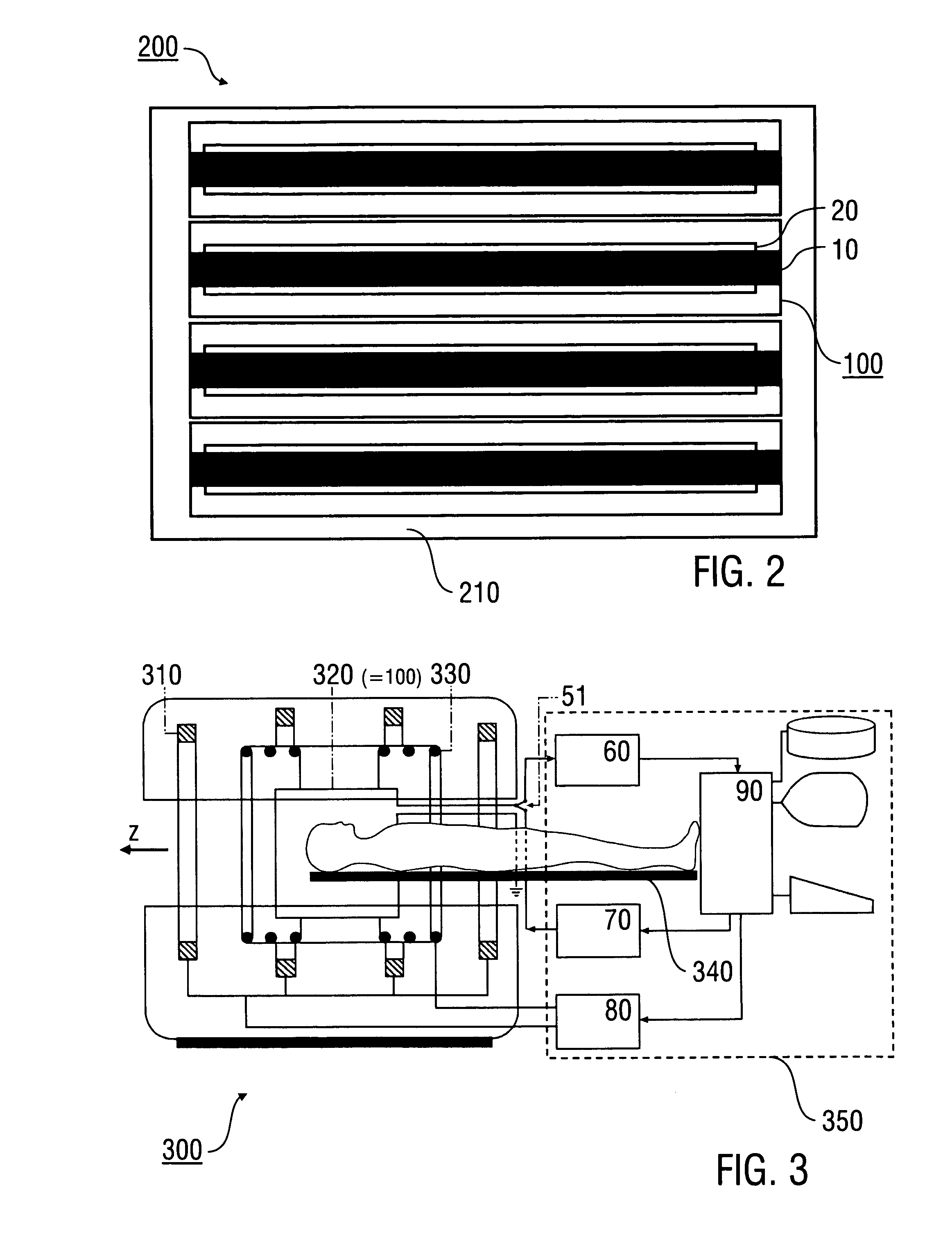
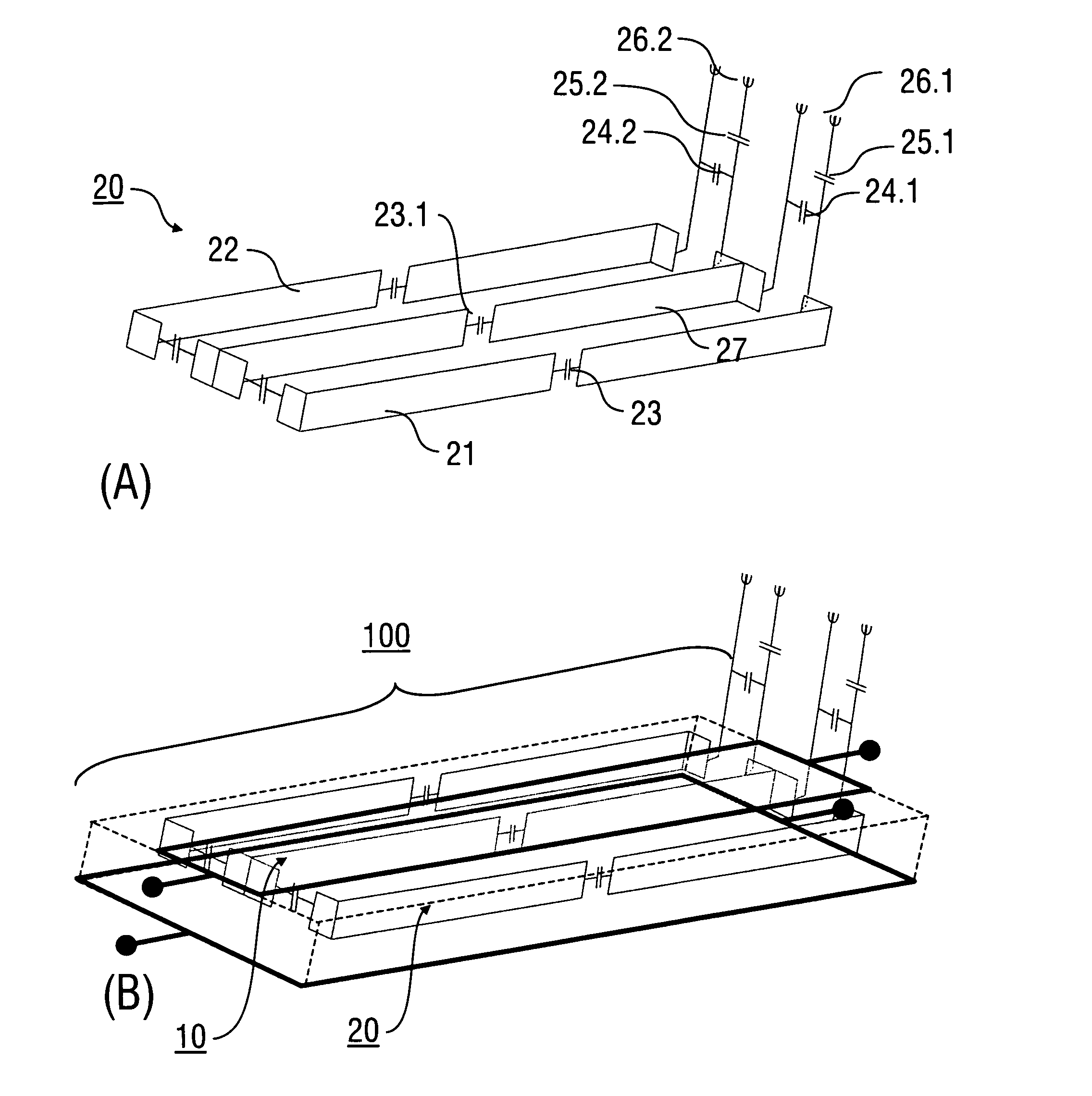
An antenna (100) for a magnetic resonance device has a predetermined sensitivity and is designed to excite and / or detect a magnetic resonance in an object under test. The antenna (100) includes a stripline resonator (10) that is equipped with at least one stripline (11), and a conductor loop arrangement (20) that adjoins the stripline resonator (10) and forms at least one conductor loop (21, 22, 28) which is interrupted by at least one capacitor (23). The sensitivity of the antenna (100) is formed by overlapping sensitivity profiles of the stripline resonator (10) and the conductor loop arrangement (20). Also described are an antenna array (200) including a plurality of antennas (100), a magnetic resonance device (300) including at least one antenna (100) or antenna array (200), and methods for magnetic resonance imaging or magnetic resonance spectroscopy.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
System and method for detecting pain and its components using magnetic resonance spectroscopy
InactiveUS7676254B2Diagnostic recording/measuringMeasurements using NMR imaging systemsMetaboliteAssessing Pain
Owner:SYDNEY UNIV OF +2
MR spectroscopy system and method for diagnosing painful and non-painful intervertebral discs
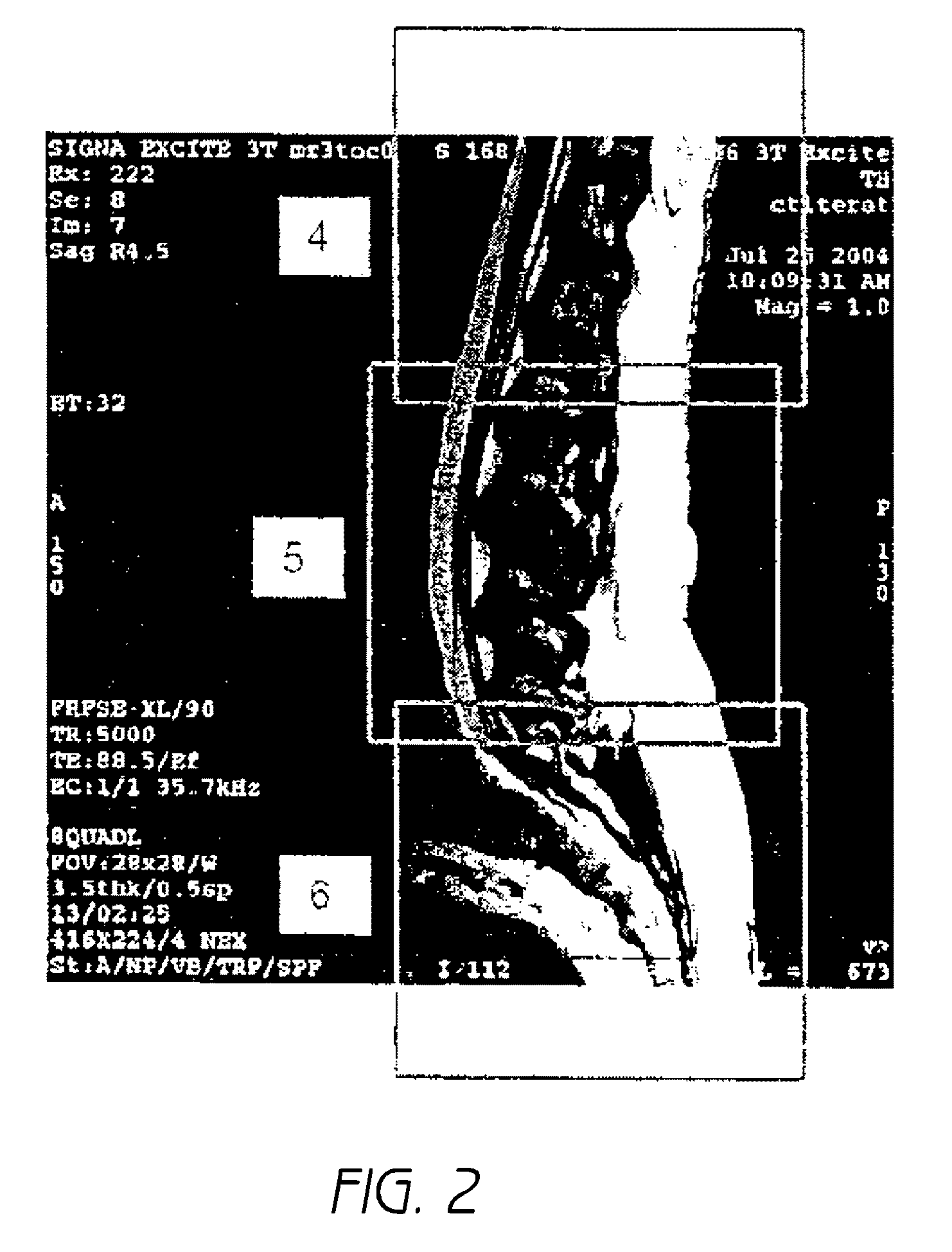
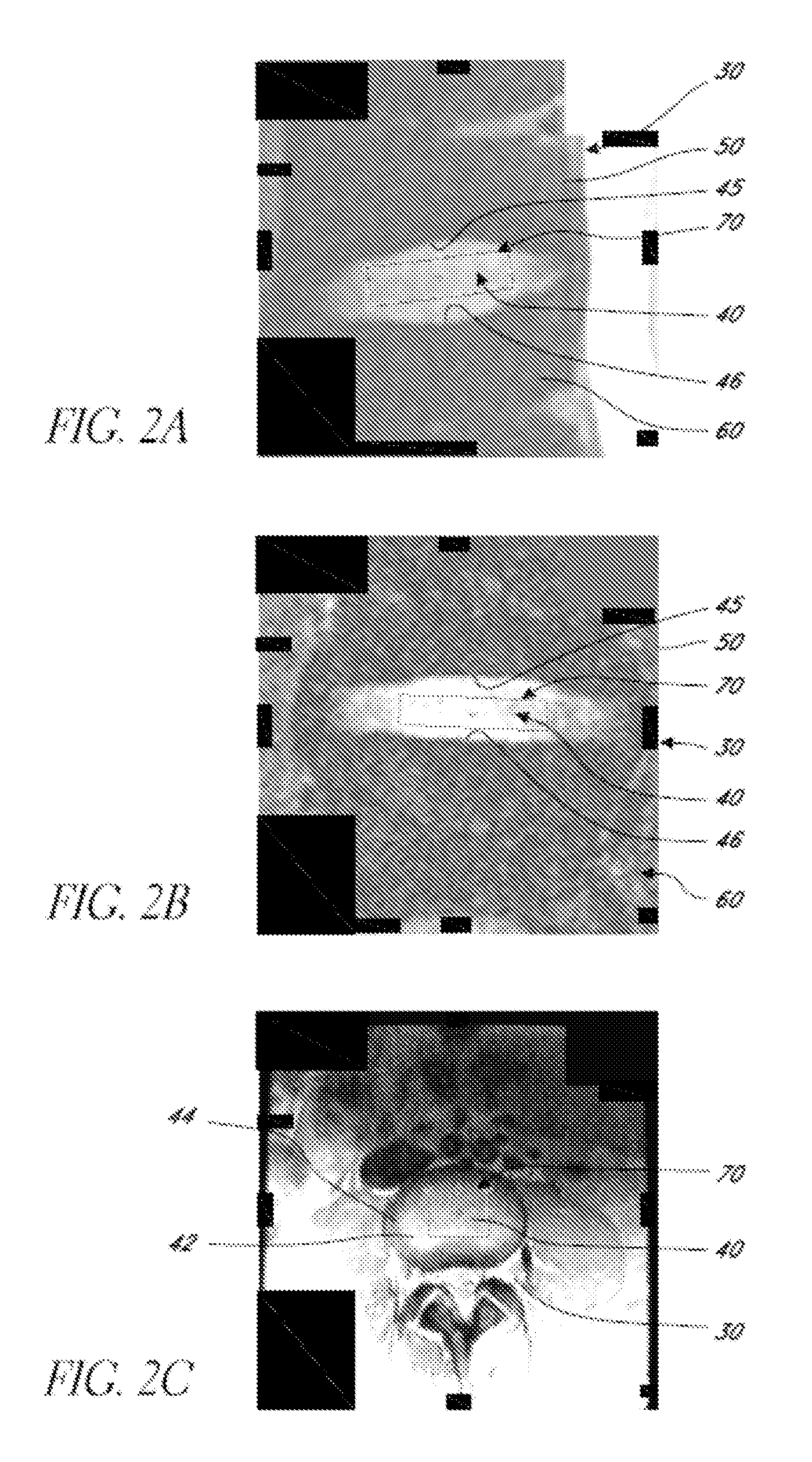
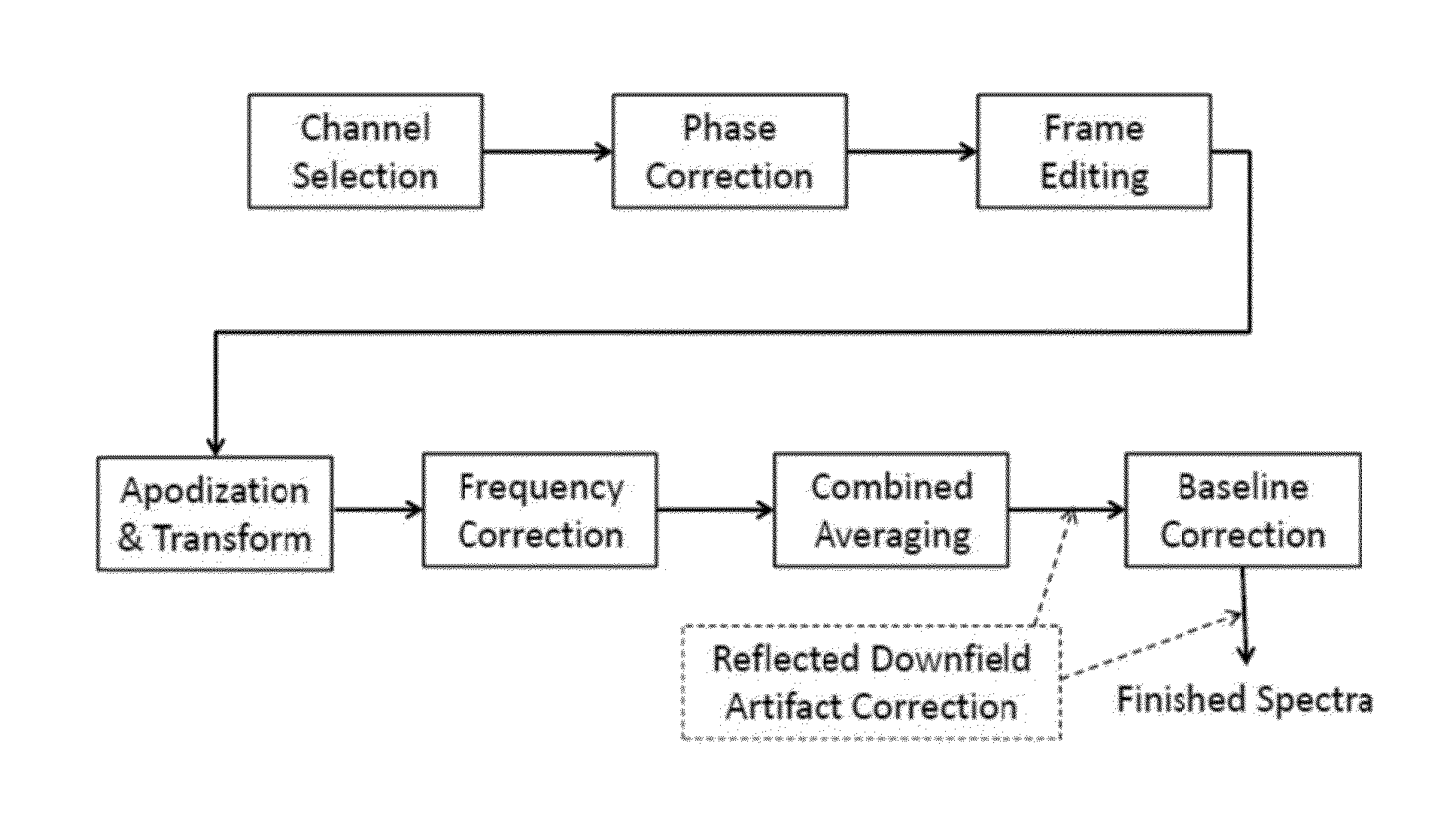
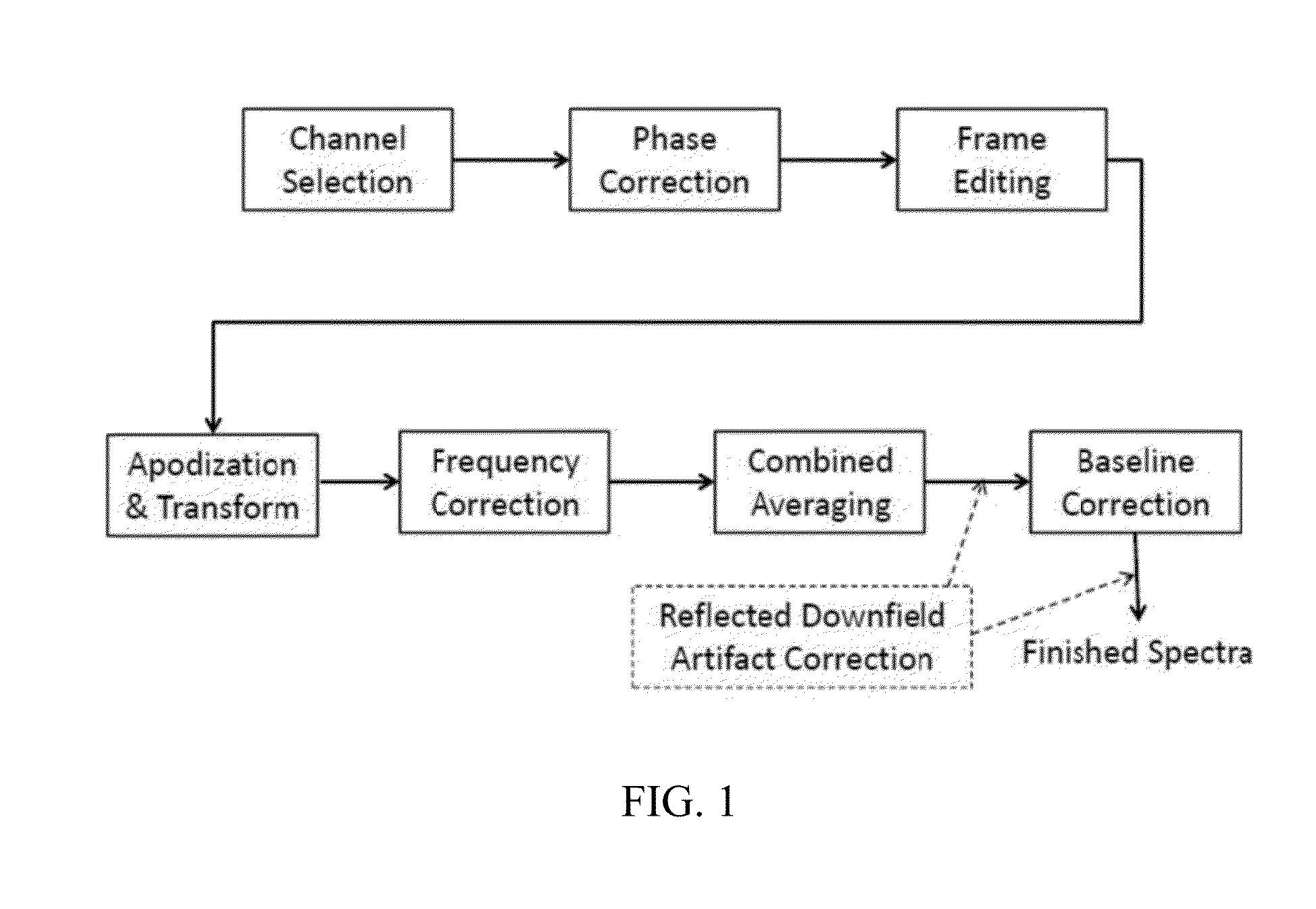
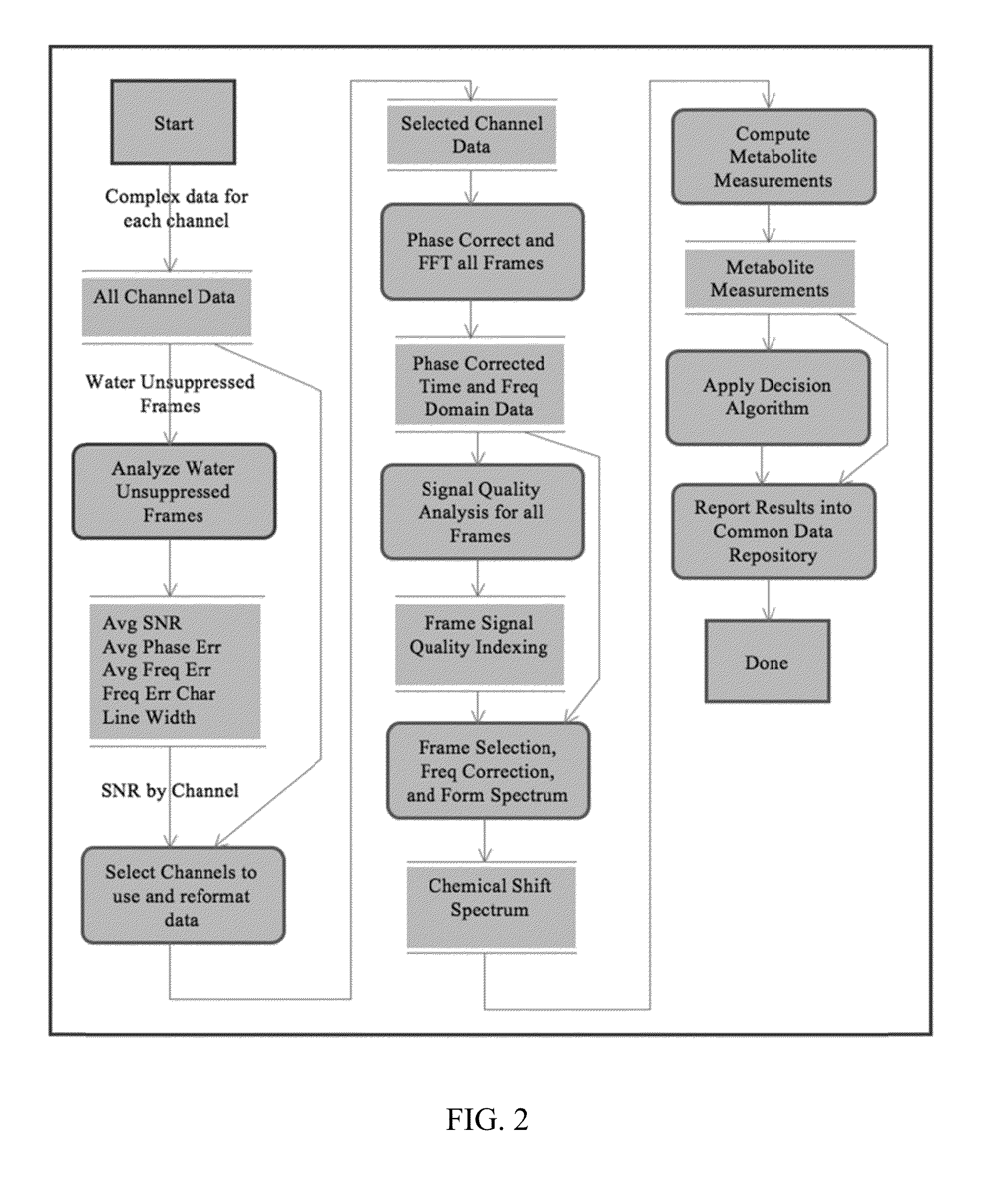
An MR Spectroscopy (MRS) system and approach is provided for diagnosing painful and non-painful discs in chronic, severe low back pain patients (DDD-MRS). A DDD-MRS pulse sequence generates and acquires DDD-MRS spectra within intervertebral disc nuclei for later signal processing & diagnostic analysis. An interfacing DDD-MRS signal processor receives output signals of the DDD-MRS spectra acquired and is configured to optimize signal-to-noise ratio (SNR) by an automated system that selectively conducts optimal channel selection, phase and frequency correction, and frame editing as appropriate for a given acquisition series. A diagnostic processor calculates a diagnostic value for the disc based upon a weighted factor set of criteria that uses MRS data extracted from the acquired and processed MRS spectra along regions associated with multiple chemicals that have been correlated to painful vs. non-painful discs. A diagnostic display provides a scaled, color coded legend and indication of results for each disc analyzed as an overlay onto a mid-sagittal T2-weighted MRI image of the lumbar spine for the patient being diagnosed. Clinical application of the embodiments provides a non-invasive, objective, pain-free, reliable approach for diagnosing painful vs. non-painful discs by simply extending and enhancing the utility of otherwise standard MRI exams of the lumbar spine.
Owner:ACLARION INC +1
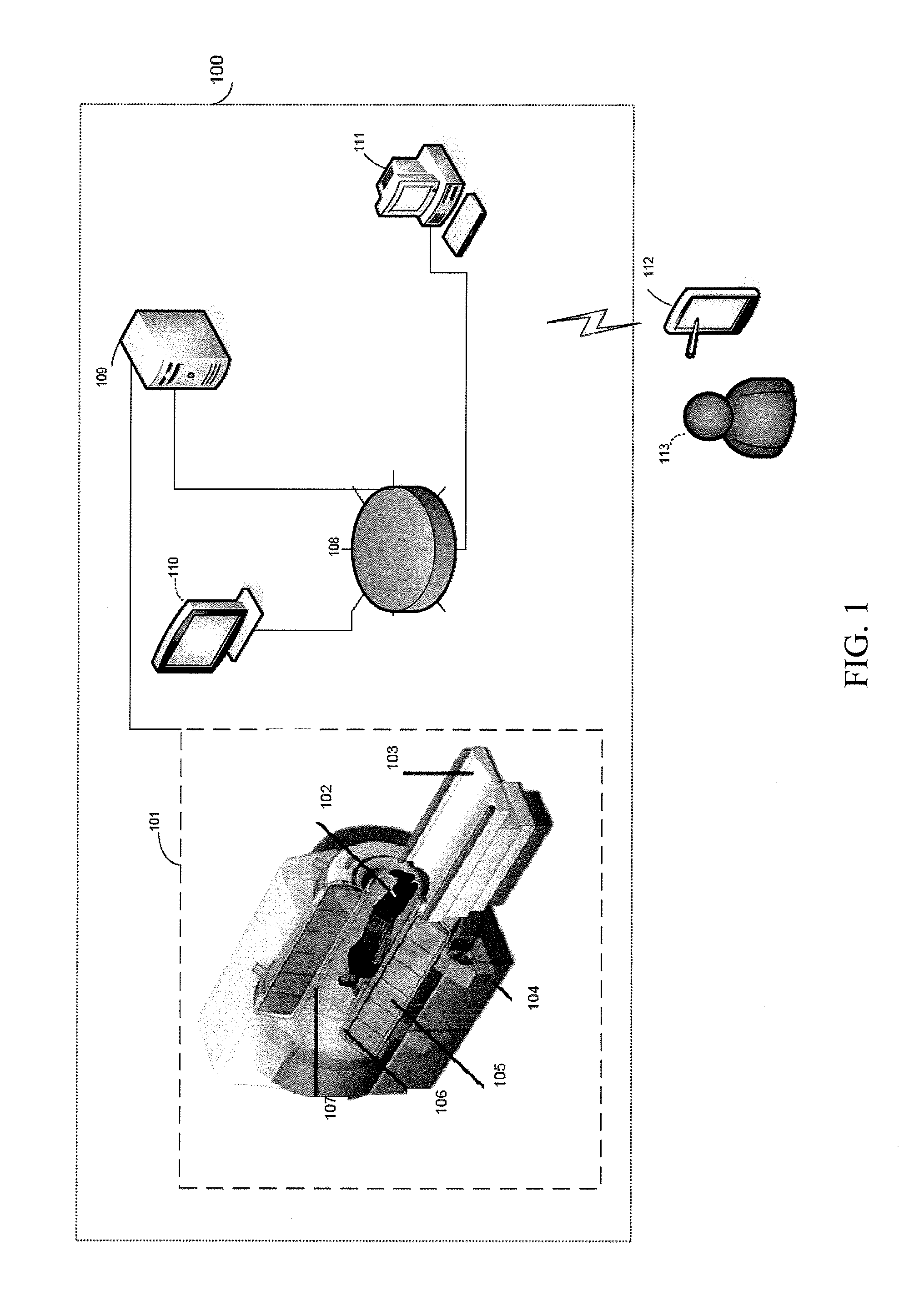
Control apparatus for controlling a therapeutic apparatus
InactiveUS20120035464A1Good effectReduced systemic side-effectsUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyMagnetic resonance spectroscopicImaging processing
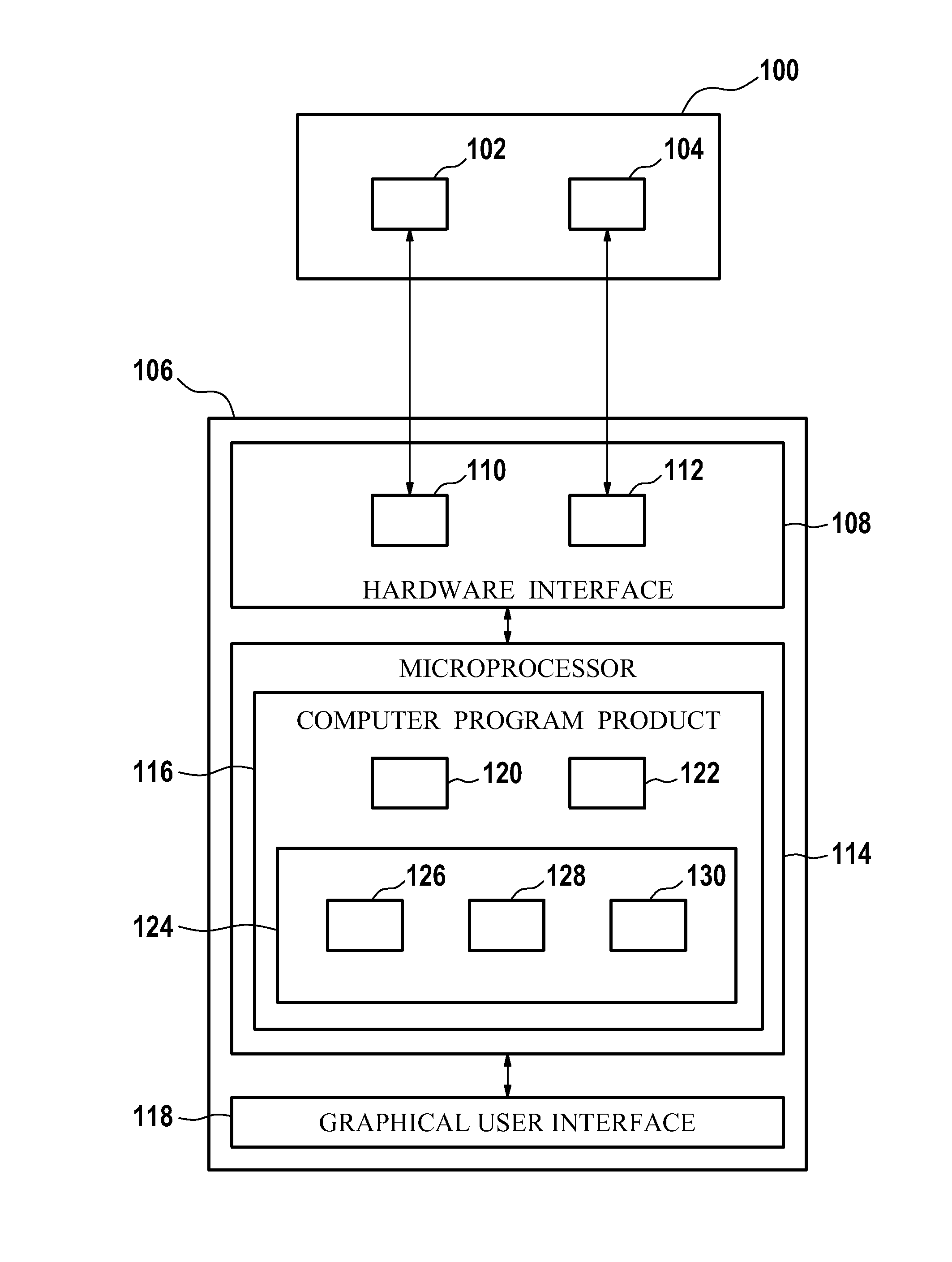
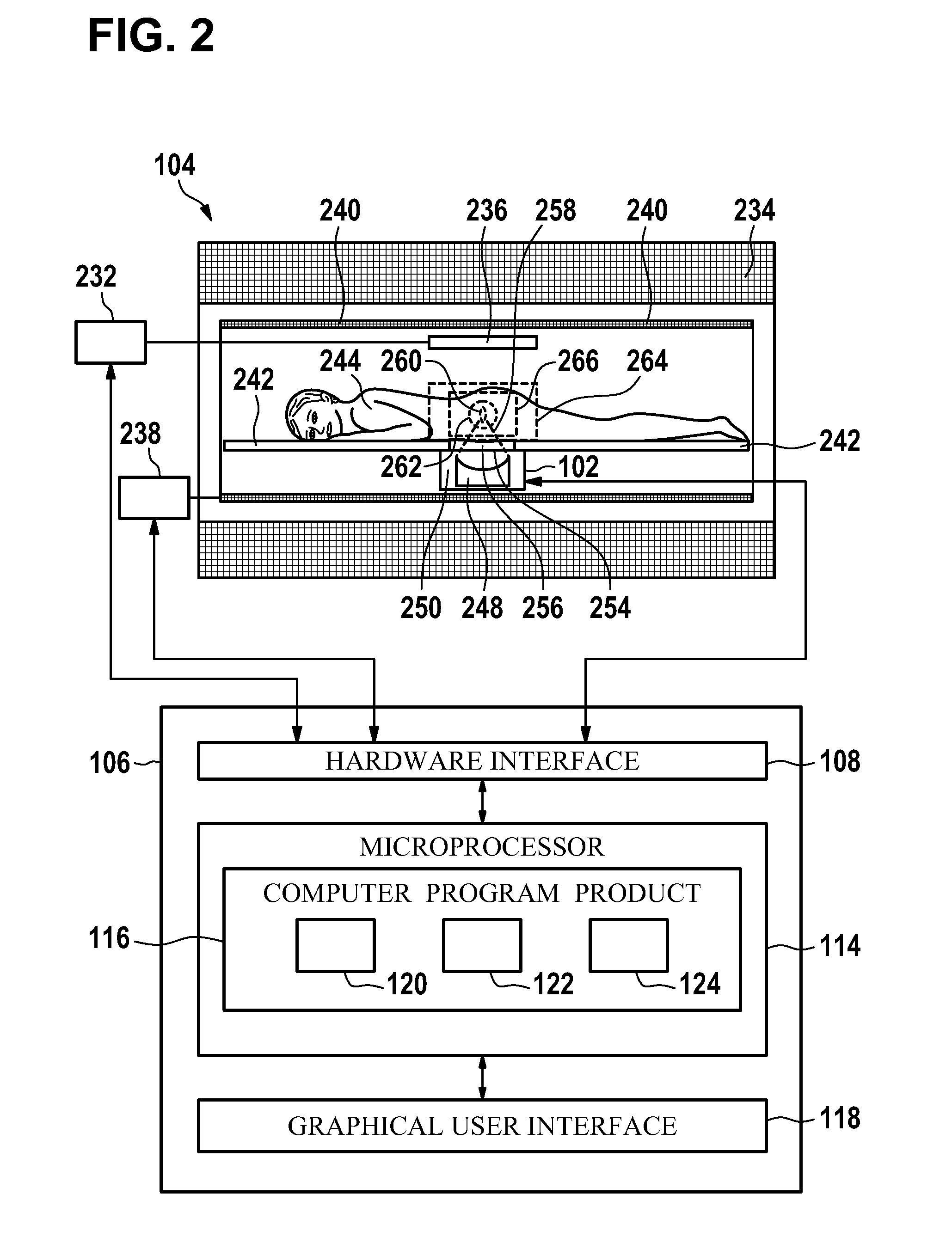
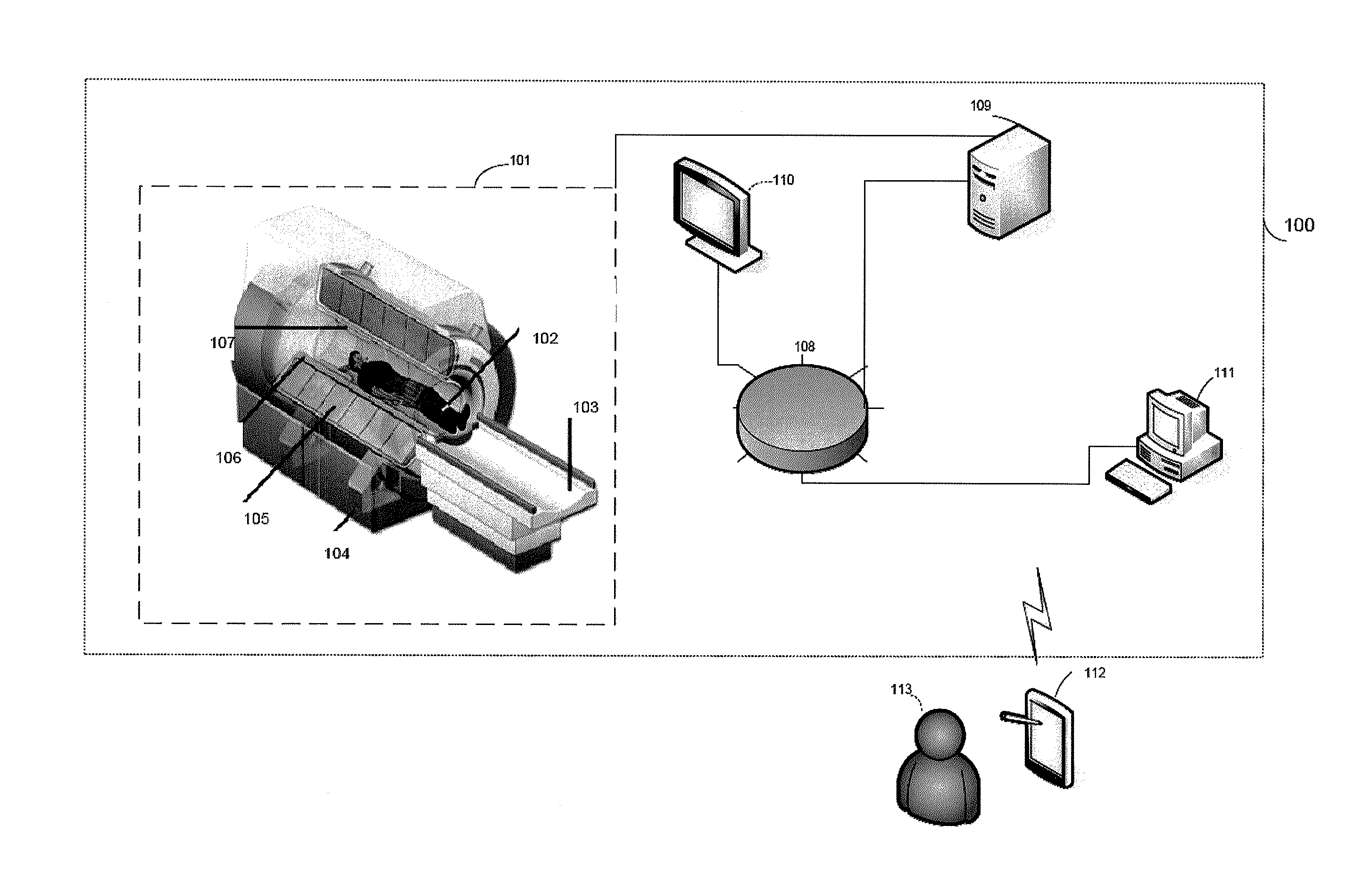
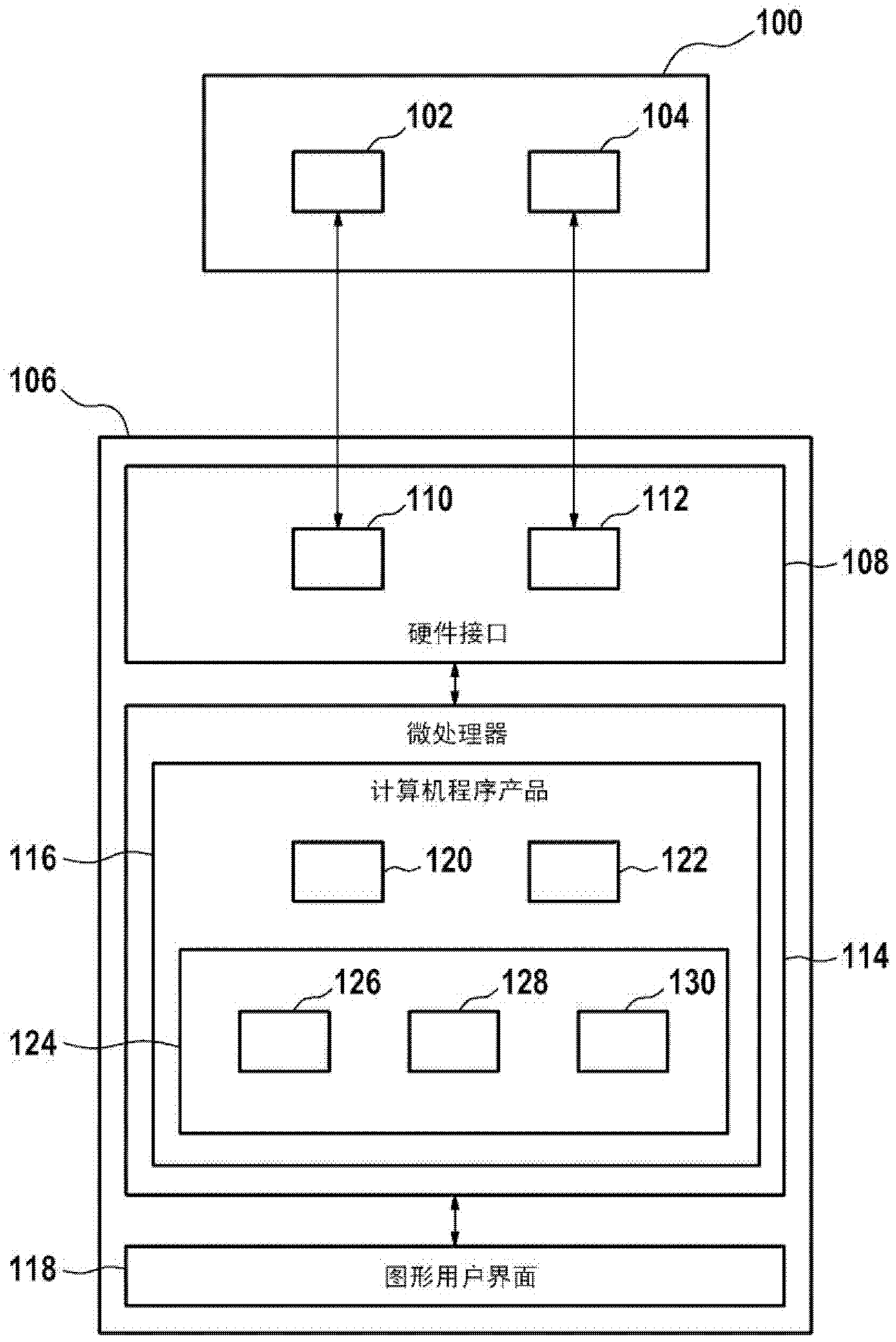
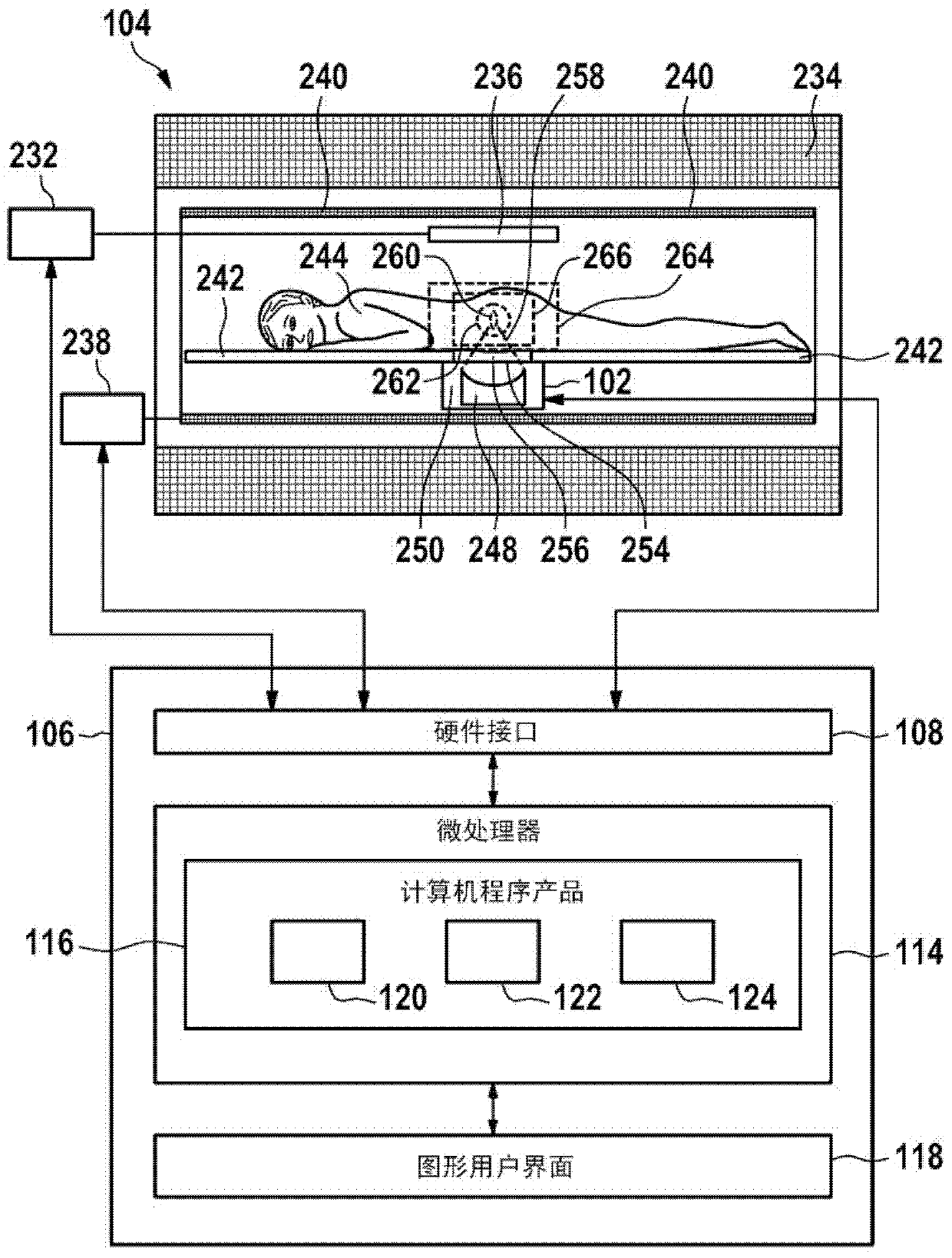
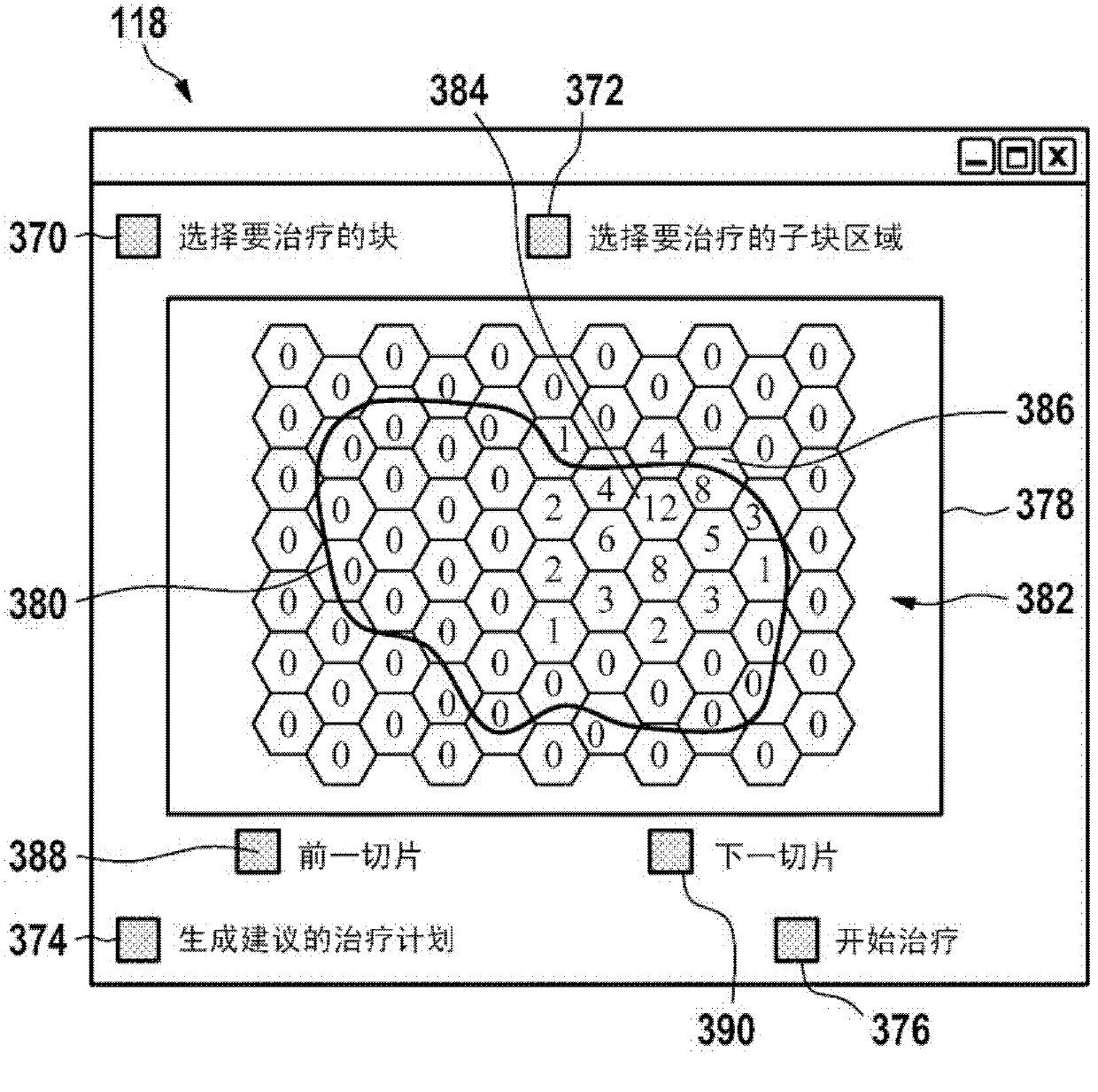
A control apparatus (106) for controlling a therapeutic apparatus (100), wherein the control apparatus comprises: —an ultrasound control interface (110) for controlling a therapeutic ultrasound system (102), —a magnetic resonance control interface (112) for controlling a magnetic resonance apparatus (104) adapted for acquiring magnetic resonance imaging data from a subject and for acquiring magnetic resonance spectroscopy data from a subject (244), —an image processing module (124, 126, 128) for generating at least one magnetic resonance imaging image (500) from the magnetic resonance imaging data and for generating at least one magnetic resonance spectroscopy map (502, 514, 516, 518, 520) from the magnetic resonance spectroscopy data, —a planning module (120) adapted for receiving the magnetic resonance imaging image and the magnetic resonance spectroscopy map and for outputting planning data (732), —a control module (122) adapted for controlling the therapeutic ultrasound system using the ultrasound control apparatus using the planning data, wherein the control module is further adapted for controlling the acquisition of the acquiring magnetic resonance imaging data and magnetic resonance spectroscopy data using the magnetic resonance control interface.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Magnetic resonance spectroscopy with real-time correction of motion and frequency drift, and real-time shimming
InactiveUS20070265520A1Suppress signalReduce sensitivityMagnetic measurementsDiagnostic recording/measuringSpectral patternObject motion
This invention relates to localized magnetic resonance spectroscopy (MRS) and to magnetic resonance spectroscopic imaging (MRSI) of the proton NMR signal, specifically to a magnetic resonance spectroscopy (MRS) method to measure a single volume of interest and to a magnetic resonance spectroscopic imaging method with at least one spectral dimension and up to three spatial dimensions. MRS and MRSI are sensitive to movement of the object to be imaged and to frequency drifts during the scan that may arise from scanner instability, field drift, respiration, and shim coil heating due to gradient switching. Inter-scan and intra-scan movement leads to line broadening and changes in spectral pattern secondary to changes in partial volume effects in localized MRS. In MRSI movement leads to ghosting artifacts across the entire spectroscopic image. For both MRS an MRSI movement changes the magnetic field inhomogeneity, which requires dynamic reshimming. Frequency drifts in MRS and MRSI degrade water suppression, prevent coherent signal averaging over the time course of the scan and interfere with gradient encoding, thus leading to a loss in localization. It is desirable to measure object movement and frequency drift and to correct object motion and frequency drift without interfering with the MRS and MRSI data acquisition.
Owner:POSSE STEFAN
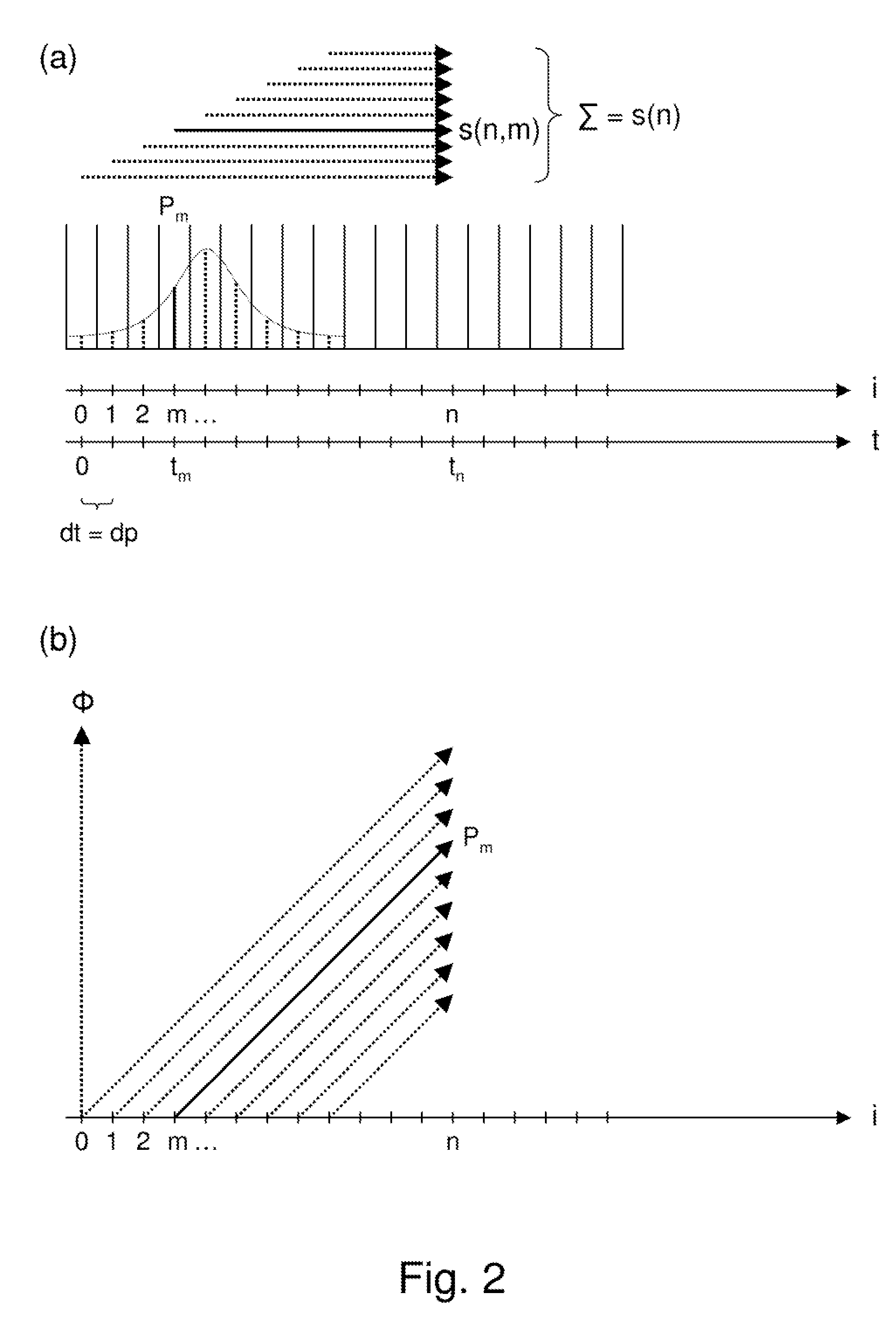
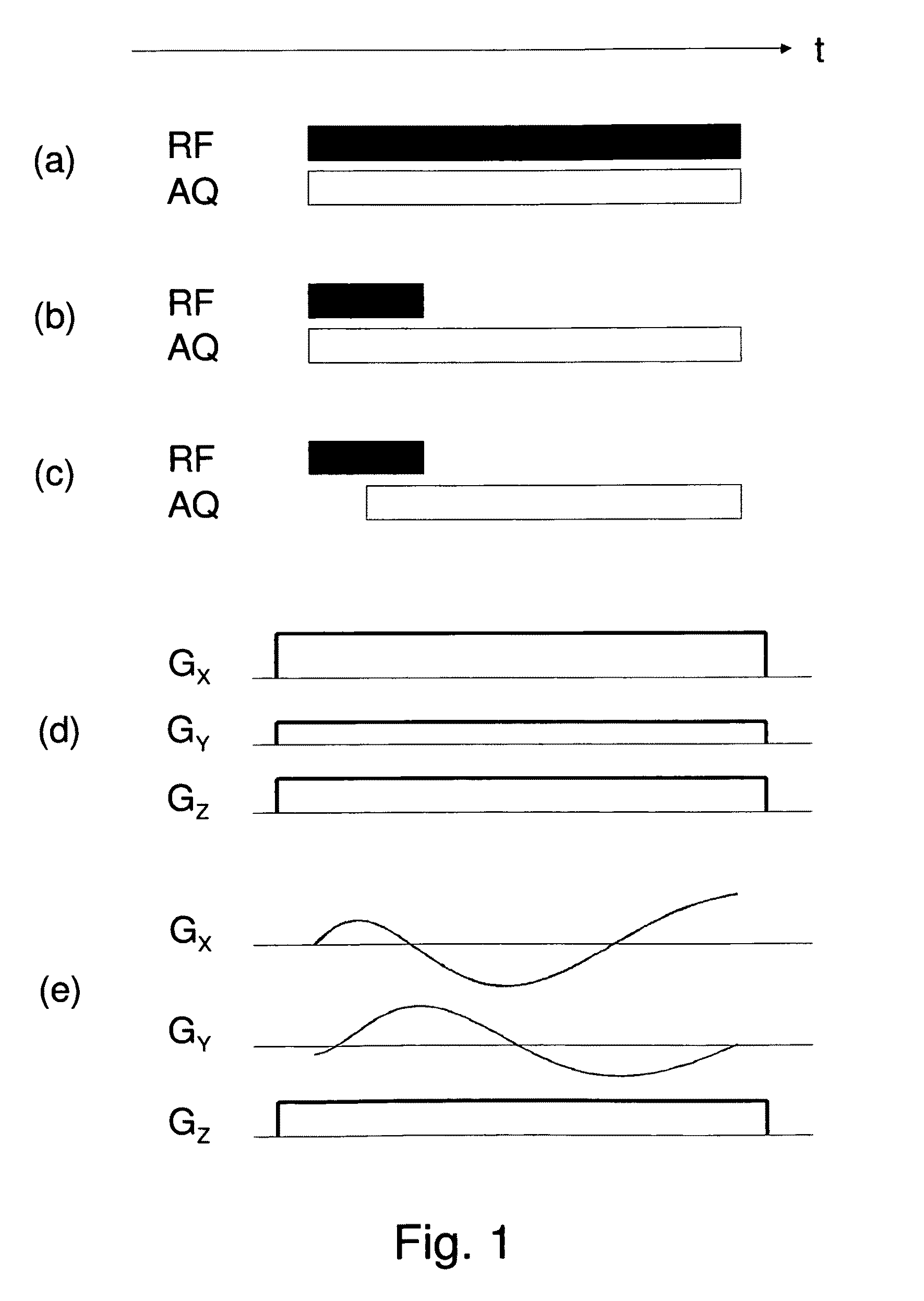
Simultaneous excitation and acquisition in magnetic resonance
ActiveUS20100244827A1Avoiding image intensity variationReduce data volumeDiagnostic recording/measuringMeasurements using NMR imaging systemsFrequency spectrumMagnetic resonance spectrometry
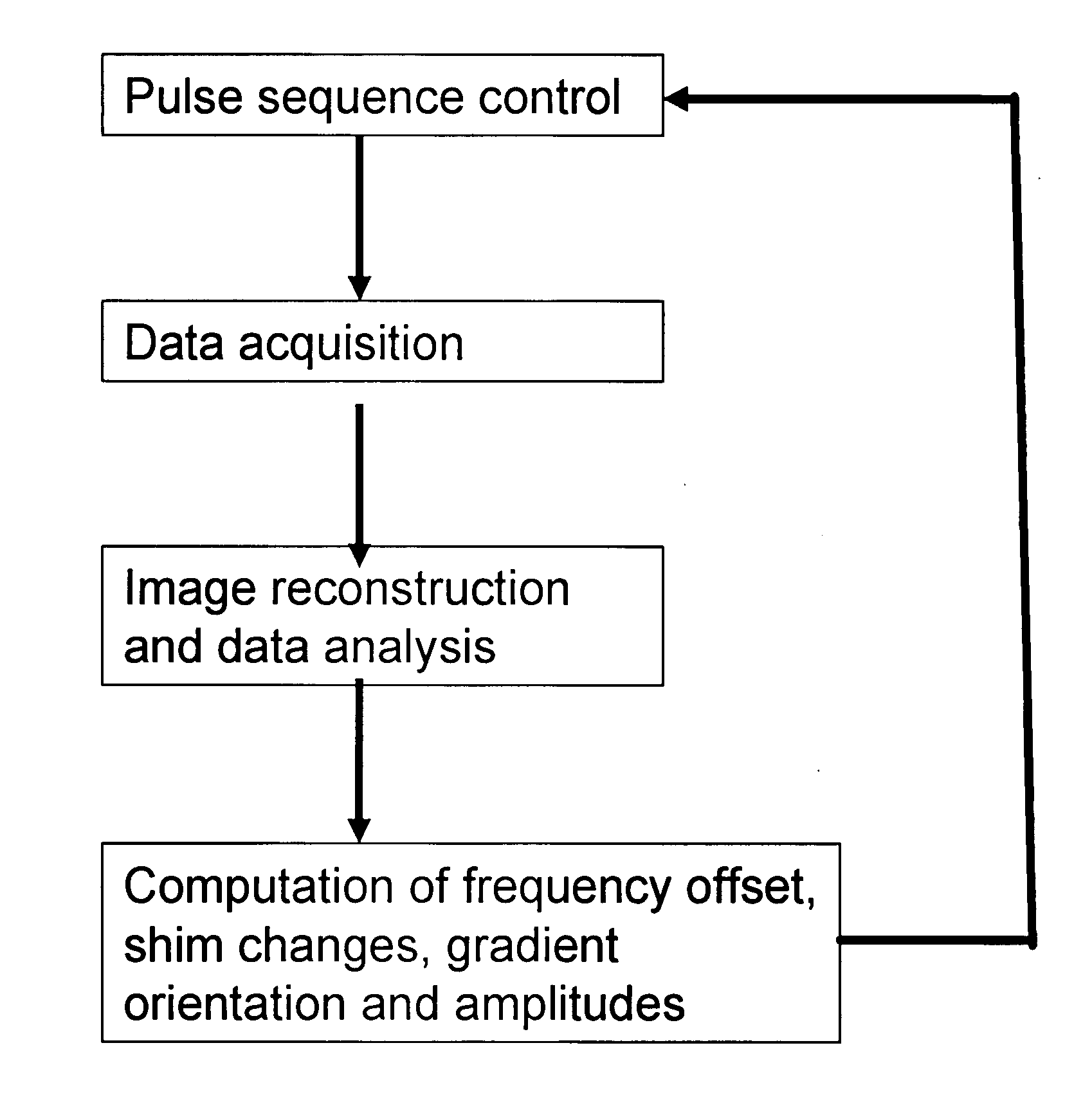
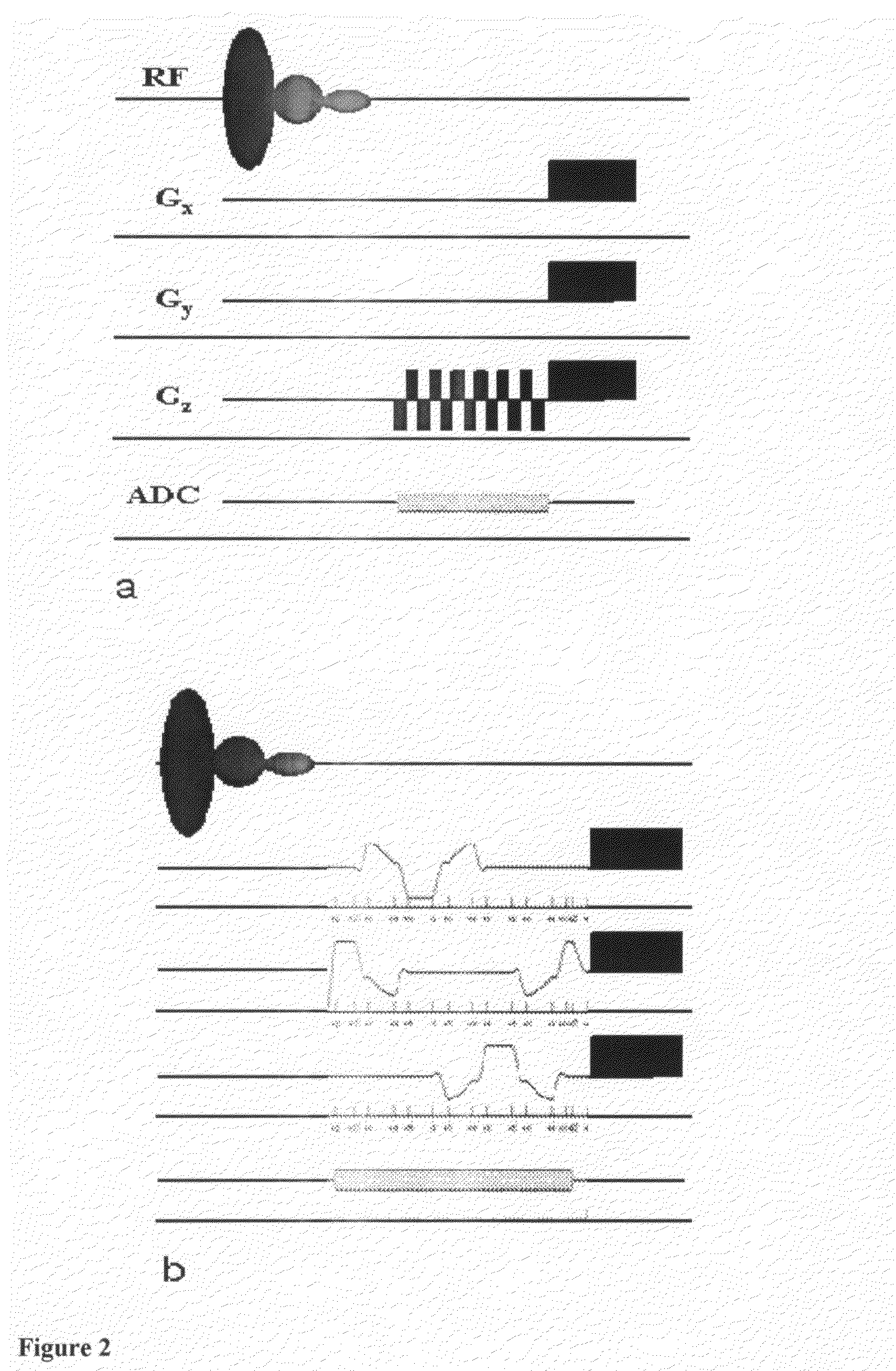
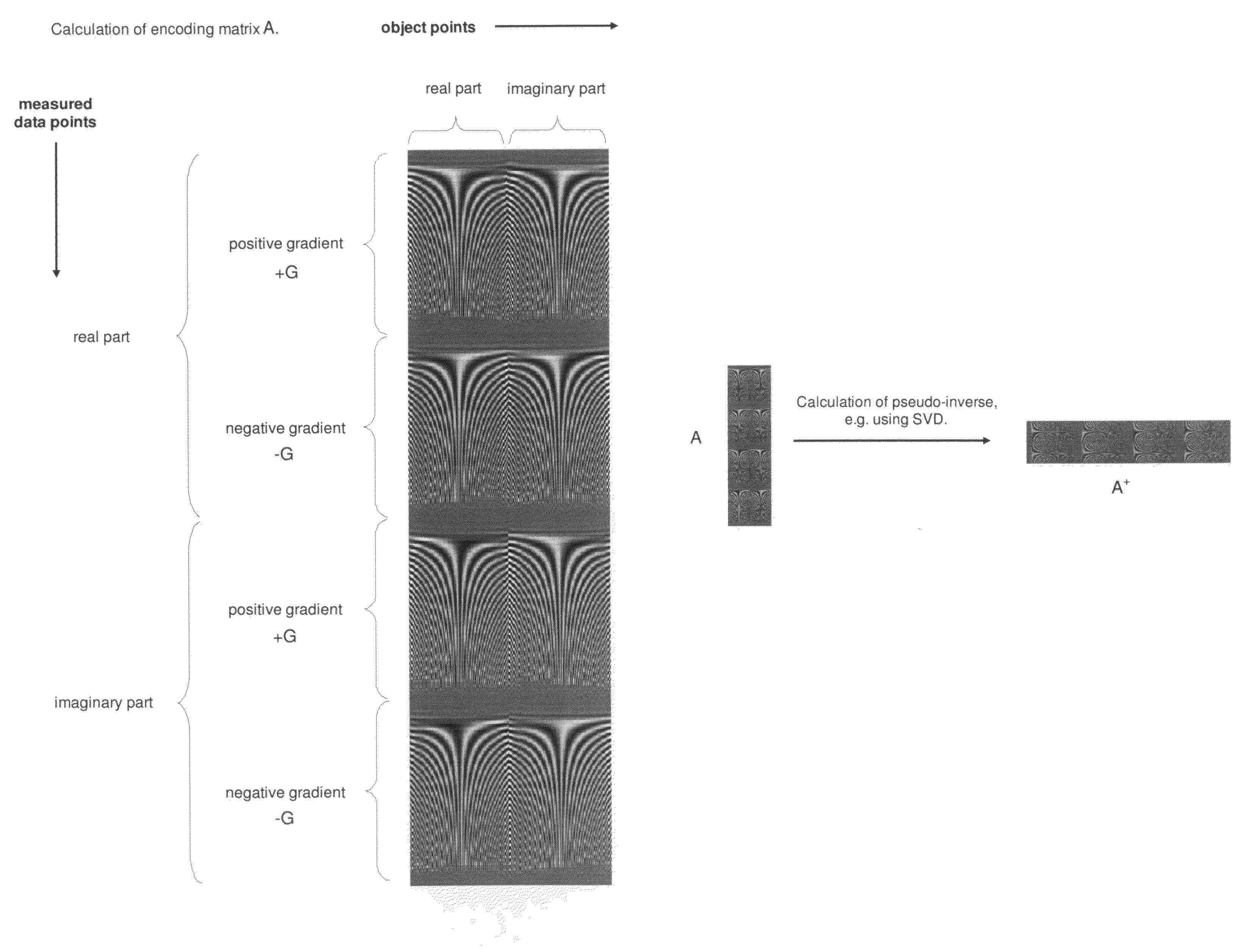
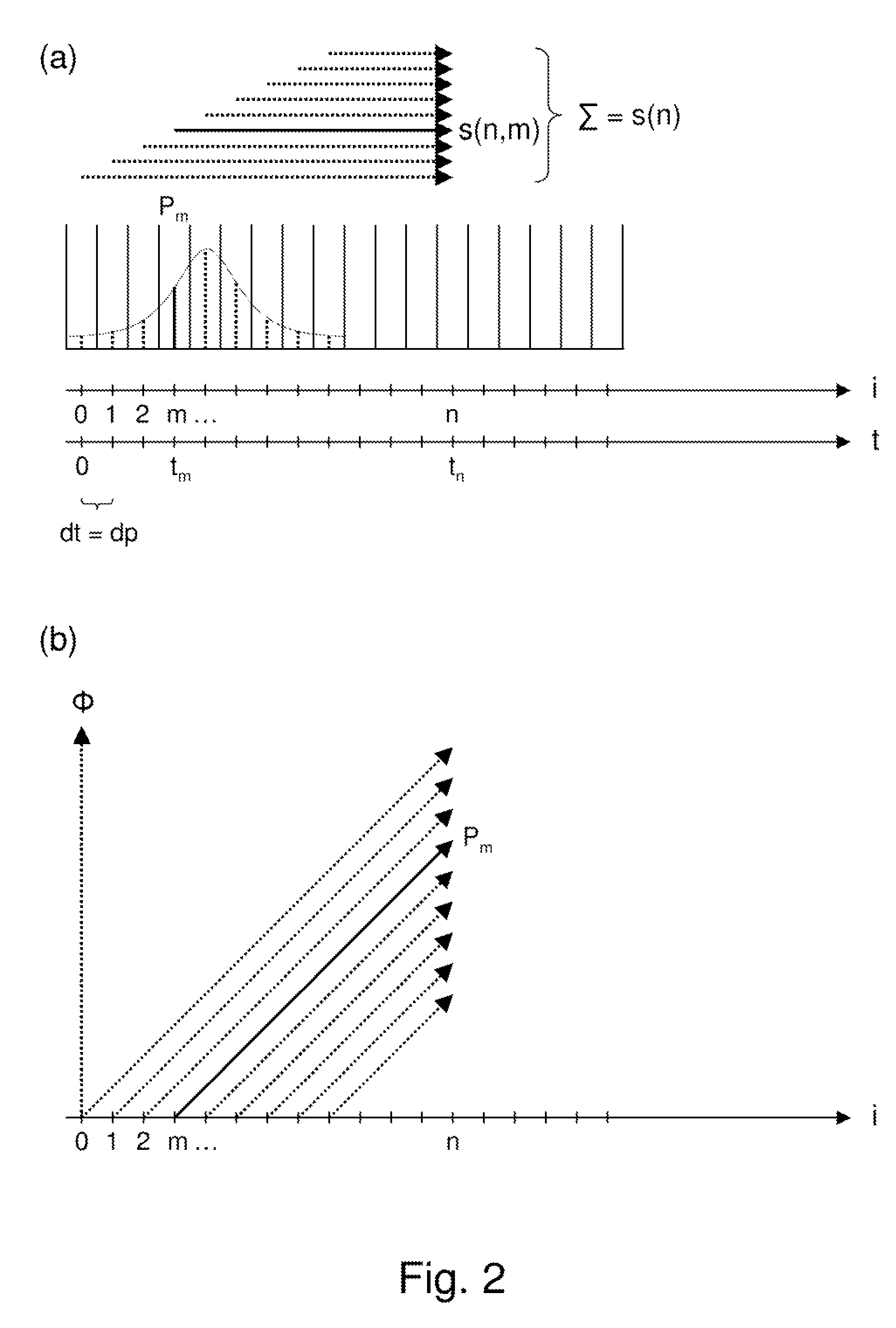
A method for magnetic resonance spectroscopy (=MRS) or magnetic resonance imaging (=MRI) in which an NMR time-domain signal is created by an RF excitation pulse applied to an object in the presence of an applied magnetic field that may depend on spatial position and / or time, the time-domain signal being generated by an excited transverse nuclear magnetisation precessing about the applied magnetic field, whereby the RF excitation pulse is adapted to cover a whole range of NMR frequencies of interest present in the object, and time-domain signal acquisition takes place during, or during and after the application of the RF excitation pulse, is characterized in that spectral or image data are reconstructed by a matrix product of a reconstruction matrix and a vector of time-domain signal points, the reconstruction matrix being an inversion of an encoding matrix Anα whose elements are calculated using the formula:Anα=∑m=0n-1PmΦ(n,m,α),wherein n is the running number of a time-domain signal point, α is the running number of a discrete image or spectral element, Pm is the m-th discrete element of the RF excitation pulse in the time-domain, and Φ(n,m,α) is the phase accrued by the transverse nuclear magnetisation related to the discrete image or spectral element a in the time between the discrete RF excitation pulse element Pm and the time-domain signal point n under the influence of the applied magnetic field. An improved method for reconstructing spectral or image data from time-domain signal acquired as describe above is thereby provided which can be used more versatilely than conventional Fourier transform.
Owner:BRUKER BIOSPIN MRI
Systems and methods for automated voxelation of regions of interest for magnetic resonance spectroscopy
A system and method for automating an appropriate voxel prescription in a uniquely definable region of interest (ROI) in a tissue of a patient is provided, such as for purpose of conducting magnetic resonance spectroscopy (MRS) in the ROI. The dimensions and coordinates of a single three dimensional rectilinear volume (voxel) within a single region of interest (ROI) are automatically identified. This is done, in some embodiments by: (1) applying statistically identified ROI search areas within a field of view (FOV); (2) image processing an MRI image to smooth the background and enhance a particular structure useful to define the ROI; (3) identifying a population of pixels that define the particular structure; (4) performing a statistical analysis of the pixel population to fit a 2D model such as an ellipsoid to the population and subsequently fit a rectilinear shape within the model; (5) repetiting elements (1) through (4) using multiple images that encompass the 3D ROI to create a 3D rectilinear shape; (6) a repetition of elements (1) through (5) for multiple ROIs with a common FOV. A manual interface may also be provided, allowing for override to replace by manual prescription, assistance to identify structures (e.g. clicking on disc levels), or modifying the automated voxel (e.g. modify location, shape, or one or more dimensions).
Owner:ACLARION INC
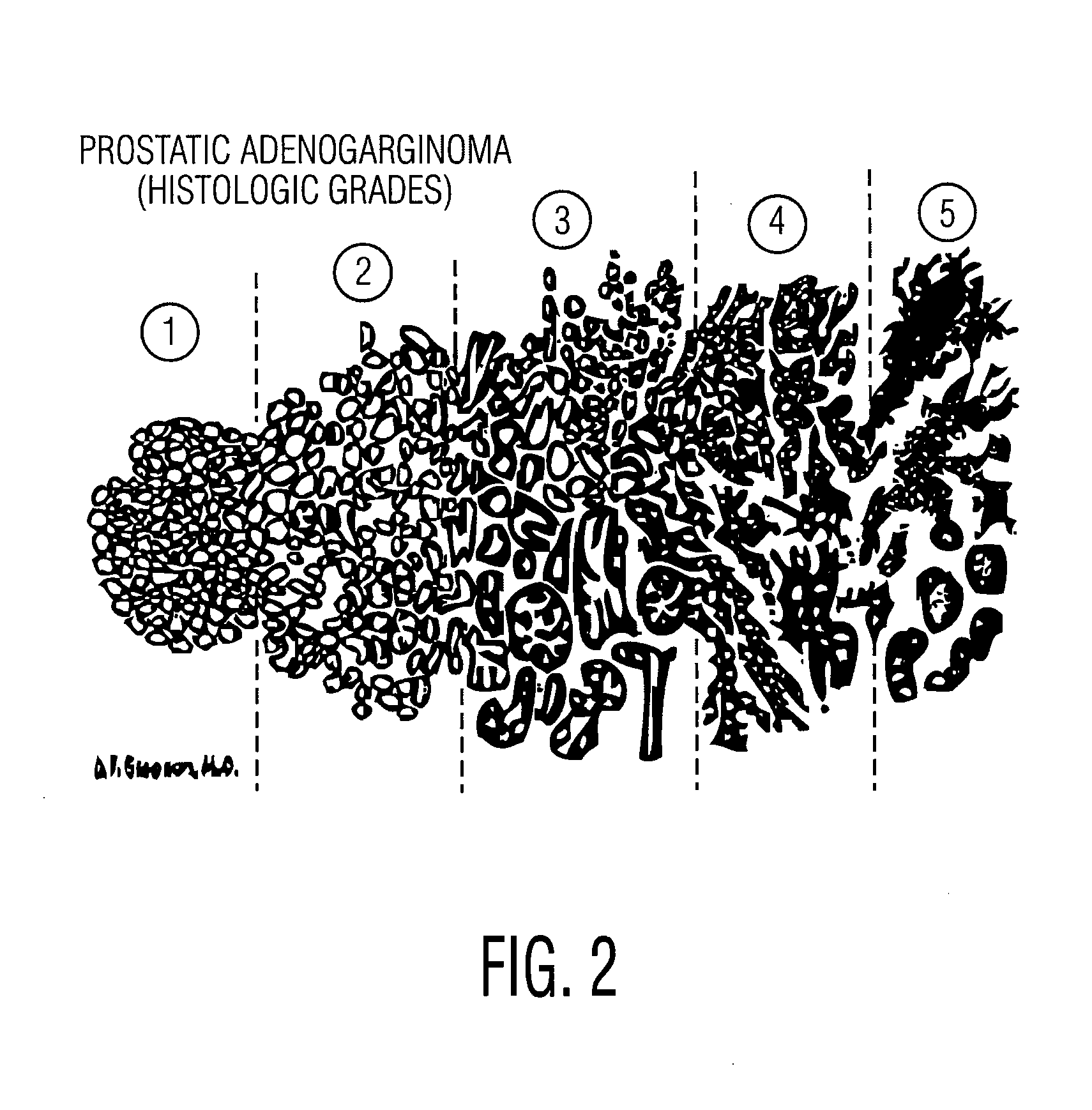
Defining quantitative signatures for different gleason grades of prostate cancer using magnetic resonance spectroscopy
InactiveUS20100169024A1Image enhancementImage analysisMagnetic resonance spectroscopicPattern recognition
A method for classifying a possible cancer from a magnetic resonance spectrographic (MRS) dataset includes extracting at least one feature from the MRS dataset as being identified with the possible cancer and embedding the extracted feature into a low dimensional space to form an embedded space. The method then clusters the embedded space into clusters representing a plurality of predetermined classes and spectrally decomposing the clusters to identify substantially significant independent metabolic signatures. The method then classifies the possible cancer as belong to one of at least two cancer classes based on the identified independent metabolic signatures.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Quick reconstruction method for under-sampling magnetic resonance spectra
ActiveCN106646303AAvoid decompositionQuick breakdownMagnetic measurementsMagnetic resonance spectroscopicSingular value decomposition
The invention discloses a quick reconstruction method for under-sampling magnetic resonance spectra, and relates to magnetic resonance spectra. The method comprises the steps of firstly, performing under-sampling on time domain signals of magnetic resonance spectra, and filling a Hankel matrix with sampling data; then reconstructing complete time domain signals by using the low rank characteristic of the matrix, and performing Fourier transformation to obtain high-quality spectra, wherein in the process, the minimization of a matrix nuclear norm item is approached by using a matrix factorization method and minimizing a matrix Frobenius norm item, so that singular value decomposition used when the nuclear norm item is solved is avoided, and the effect of accelerating reconstruction is achieved. The method is excellent in effect and easy to operate, and can be applied to under-sampling magnetic resonance spectrum reconstruction of one, two and higher dimensions.
Owner:XIAMEN UNIV OF TECH
Simultaneous excitation and acquisition in magnetic resonance
ActiveUS8432165B2Reduce complexityMagnetic measurementsDiagnostic recording/measuringFrequency spectrumData acquisition
Owner:BRUKER BIOSPIN MRI
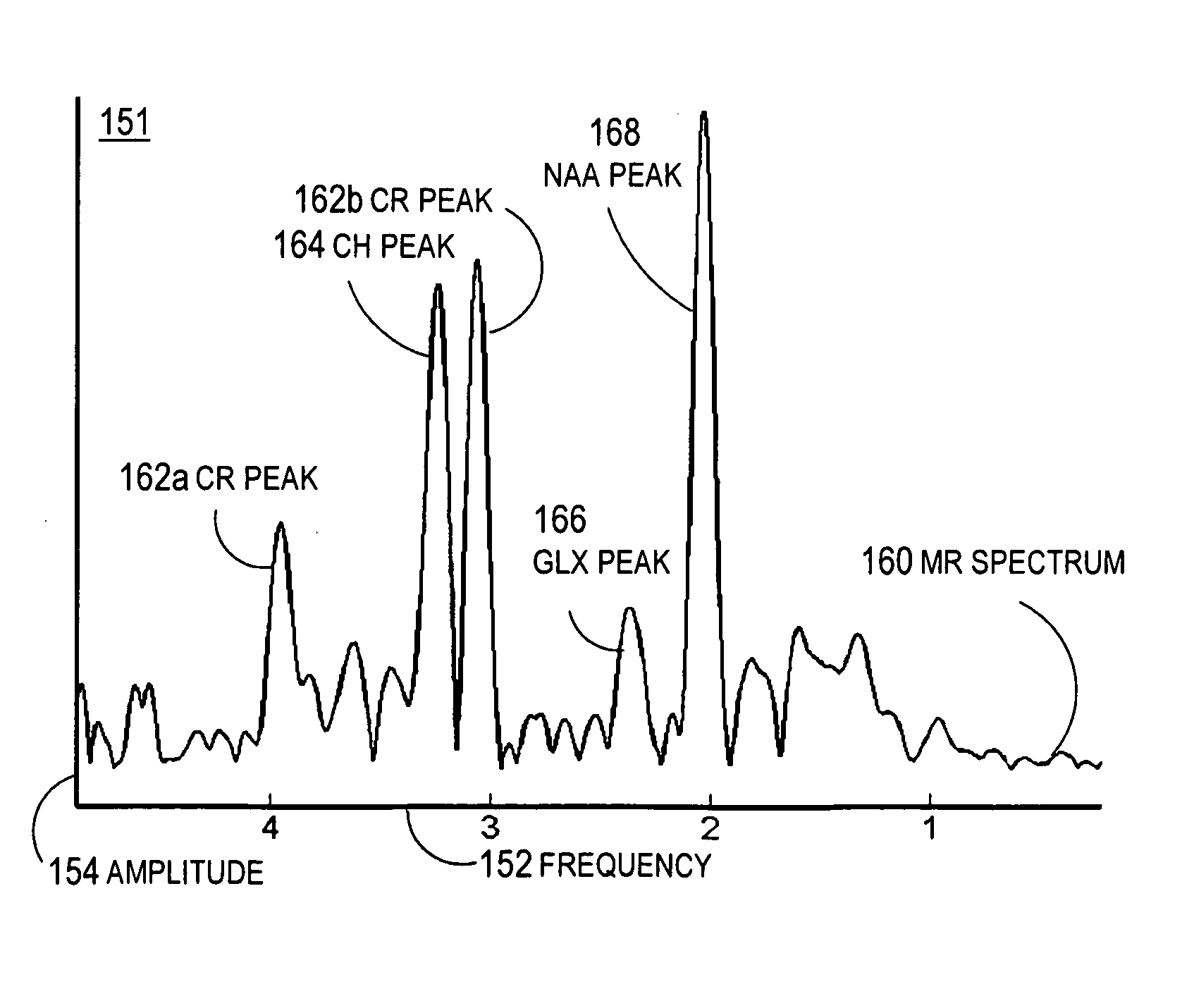
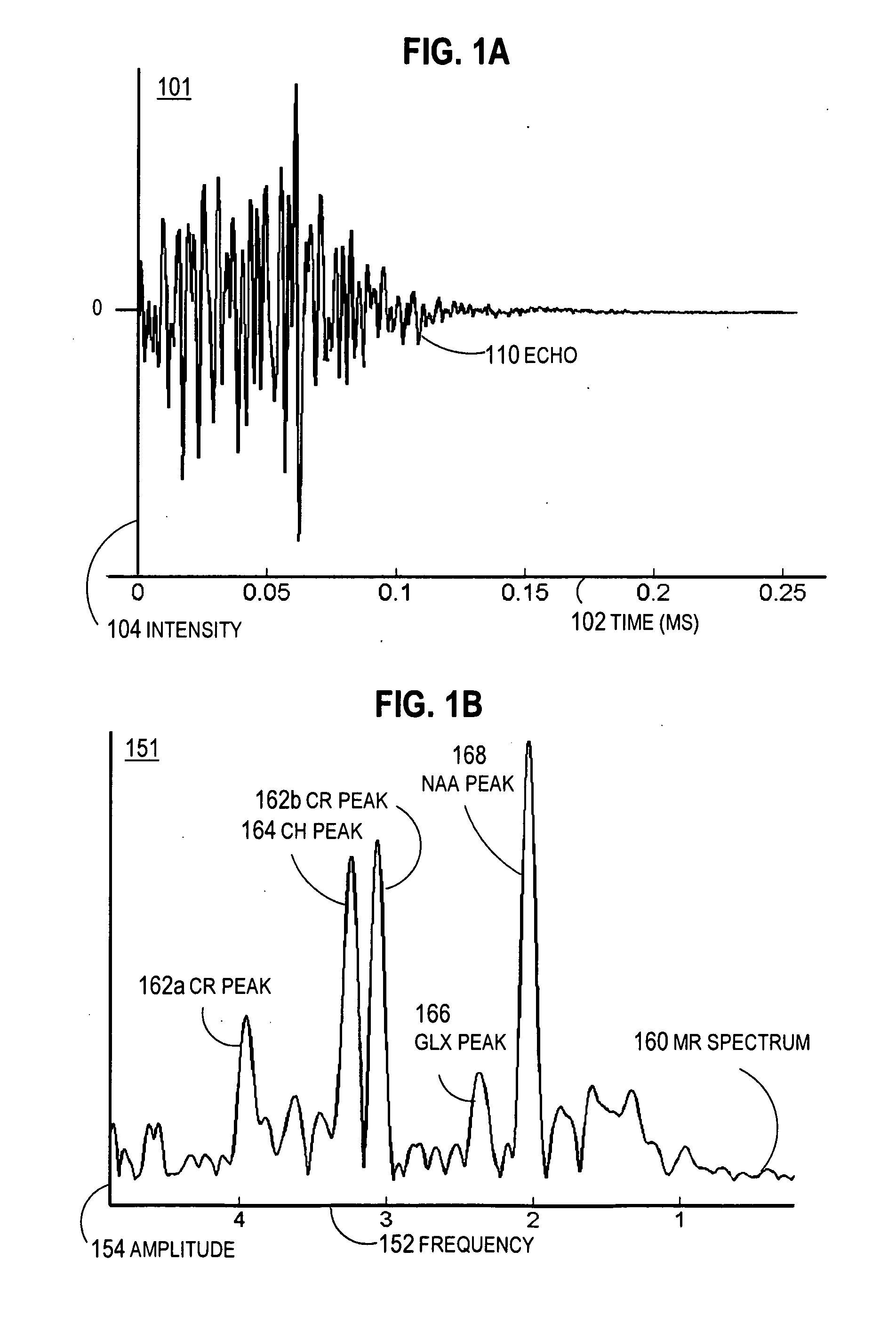
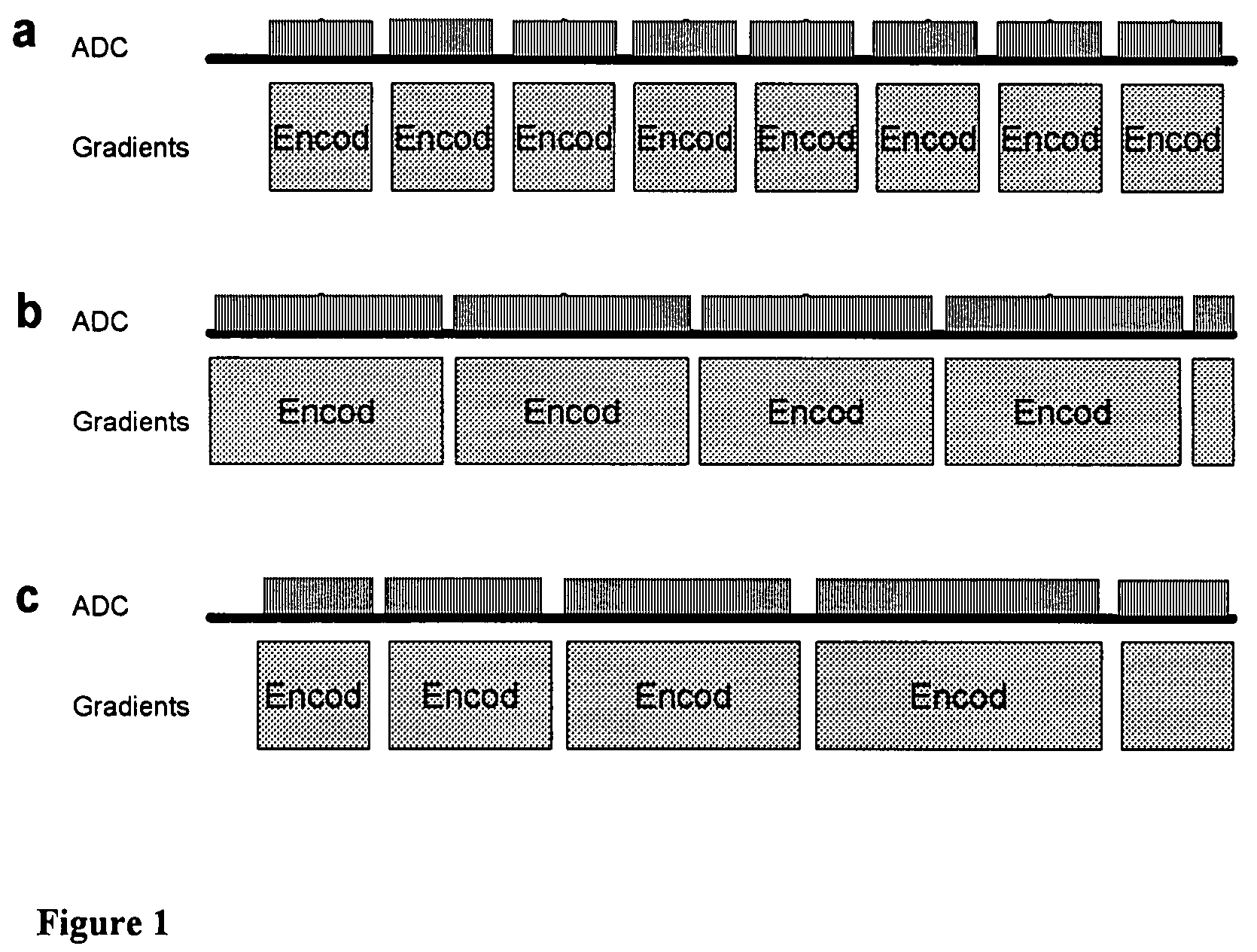
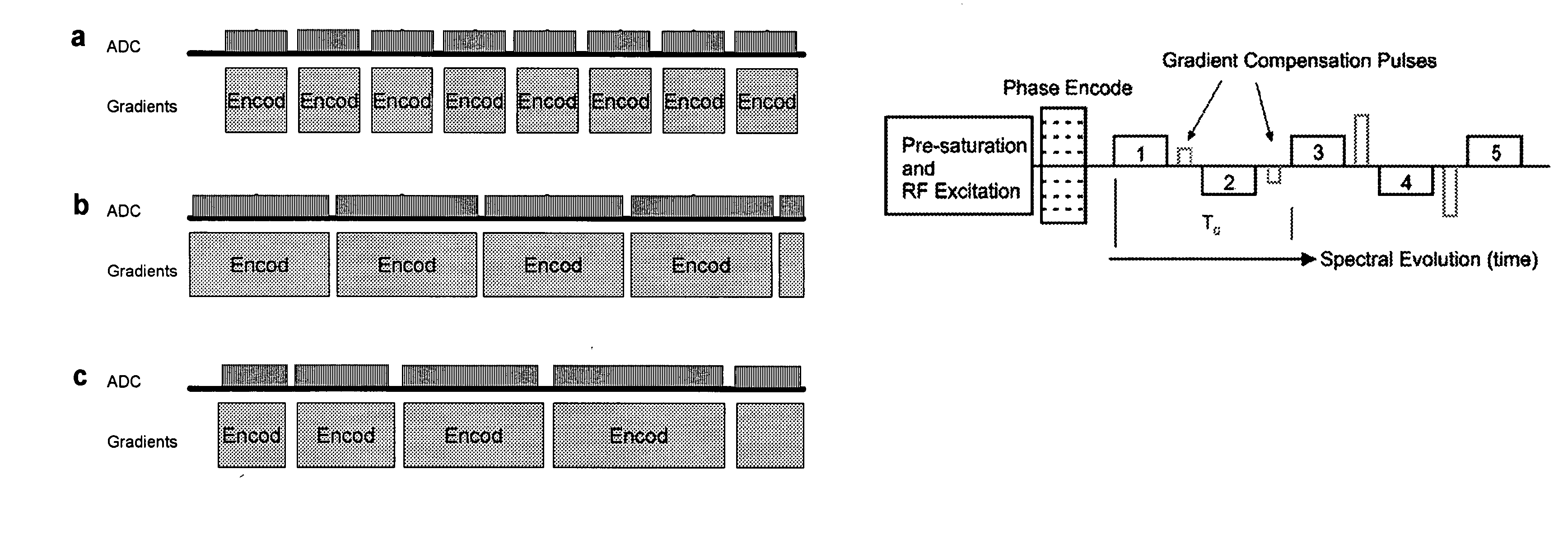
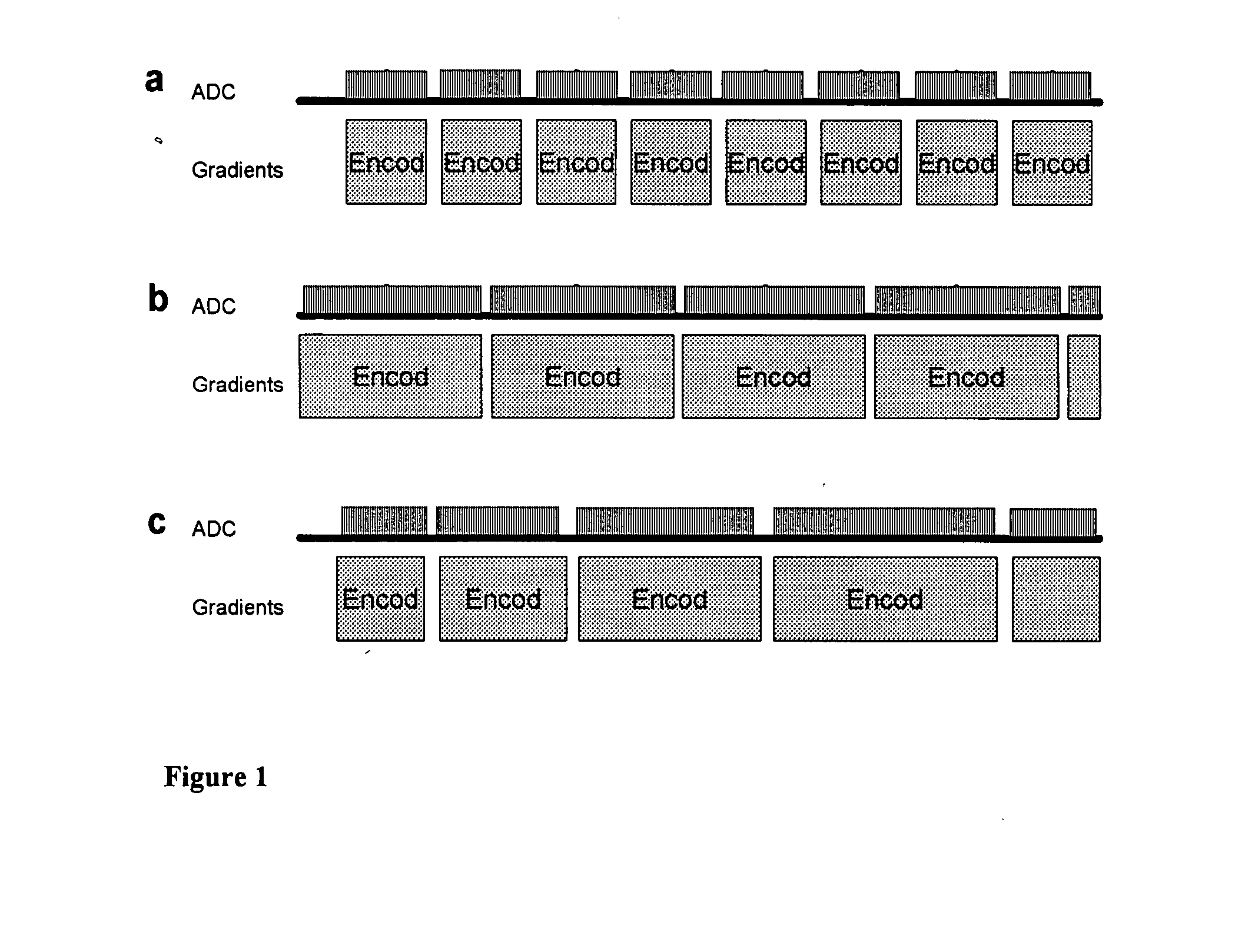
Magnetic resonance spectroscopy pulse sequence, acquisition, and processing system and method
ActiveUS8965094B2Enhanced frequency shift artifact correctionEliminate the effects ofImage analysisDiagnostics using spectroscopyMagnetic resonance spectroscopicMultiple frame
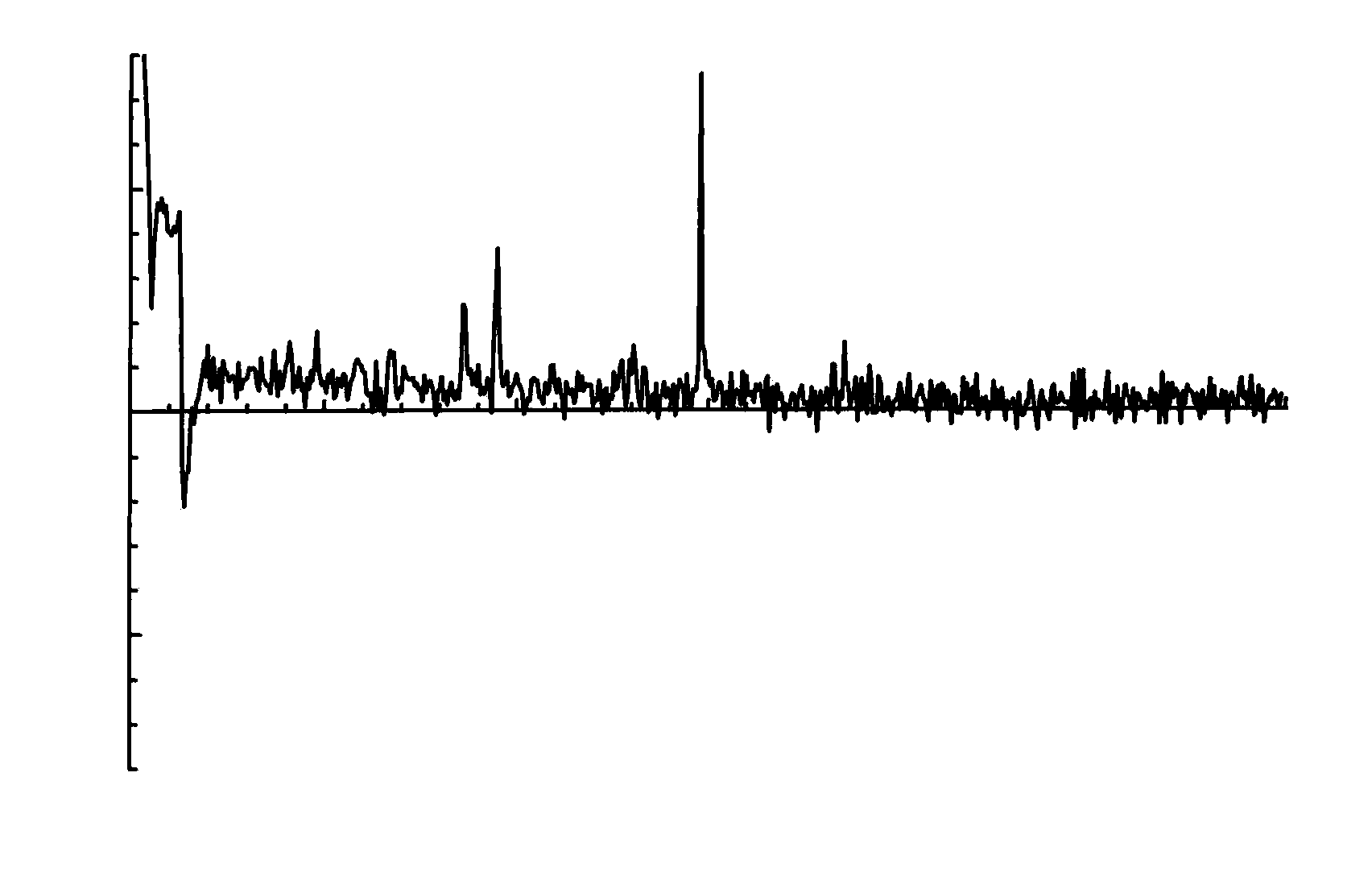
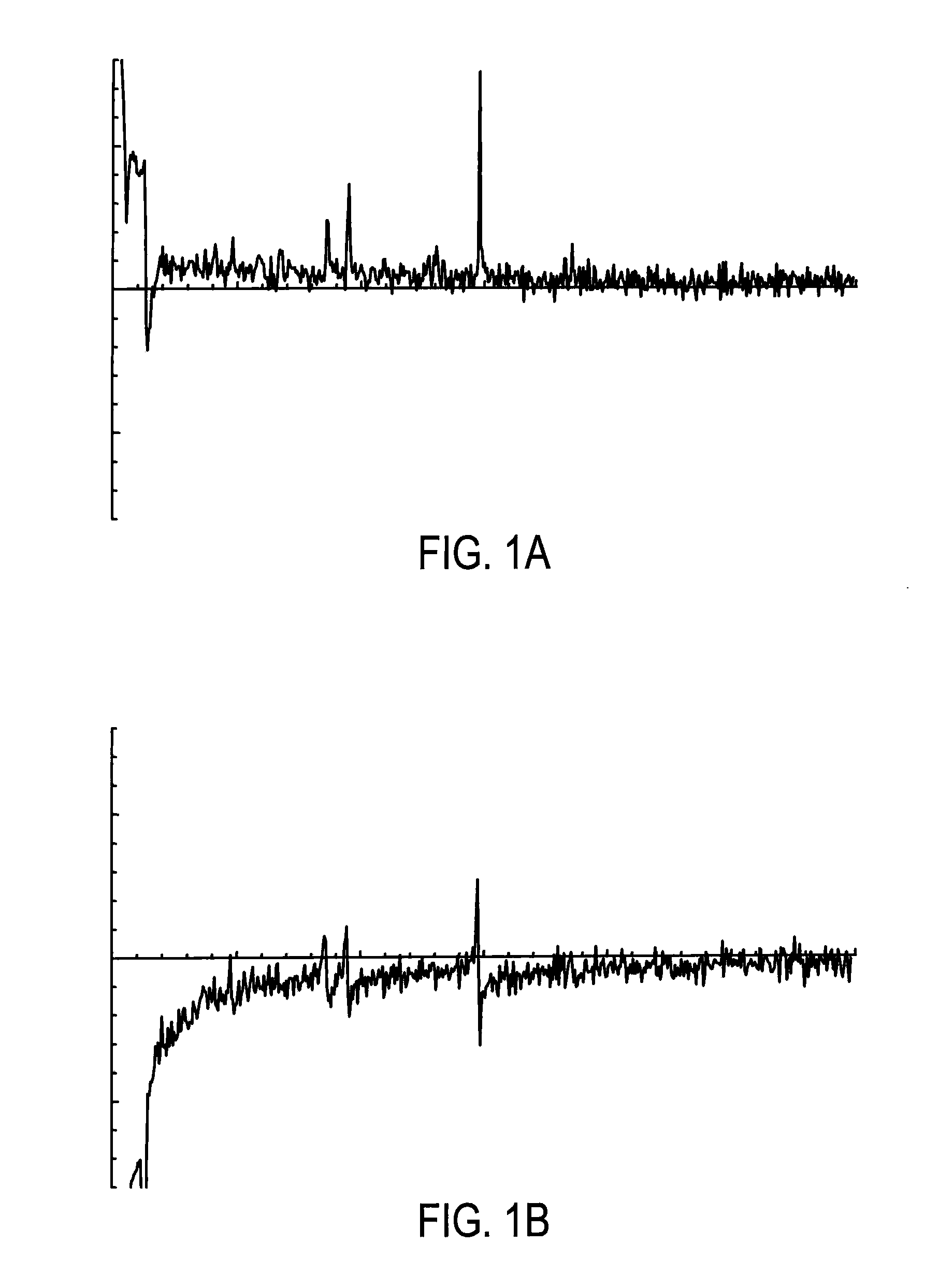
Systems and methods are provided for processing a set of multiple serially acquired magnetic resonance spectroscopy (MRS) free induction decay (FID) frames from a multi-frame MRS acquisition series from a region of interest (ROI) in a subject, and for providing a post-processed MRS spectrum. Processing parameters are dynamically varied while measuring results to determine the optimal post-processed results. Spectral regions opposite water from chemical regions of interest are evaluated and used in at least one processing operation. Frequency shift error is estimated via spectral correlation between free induction decay (FID) frames and a reference spectrum. Multiple groups of FID frames within the acquired set are identified to different phases corresponding with a phase step cycle of the acquisition. Baseline correction is also performed via rank order filter (ROF) estimate and a polynomial fit. Sections of the ROF may be excluded from the polynomial fit, such as for example sections determined to be associated with relevant spectral peaks.
Owner:ACLARION INC
System and method for phase offset and time delay correction in magnetic resonance spectroscopy data
ActiveUS20110066025A1High resolutionImprove signal-to-noise ratioMagnetic measurementsDiagnostic recording/measuringData setFrequency spectrum
A method is provided for producing, with a magnetic resonance (MR) system, a nuclear magnetic resonance spectrum that has been corrected for errors arising from phase offsets and time shifts in the acquired spectroscopic data. A spectroscopic data set includes temporal information indicative of the underlying phase offsets and time shifts. In general, this temporal information is utilized to correct for errors in the acquired data. As a result, a plurality of acquired spectroscopic data sets are more accurately combined by first individually correcting each spectroscopic data set for such errors. Exemplary sources of the phase offsets and time shifts include the physical separation between a volume of interest and the detectors in the MRI system. Using the temporal information, T2 decay in the produced spectrum is also corrected.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Stripline antenna and antenna array for a magnetic resonance device
InactiveUS8581588B2Antenna structure is simpleSimple structureMeasurements using NMR imaging systemsElectric/magnetic detectionMagnetic resonance spectroscopicElectrical conductor
An antenna (100) for a magnetic resonance device has a predetermined sensitivity and is designed to excite and / or detect a magnetic resonance in an object under test. The antenna (100) includes a stripline resonator (10) that is equipped with at least one stripline (11), and a conductor loop arrangement (20) that adjoins the stripline resonator (10) and forms at least one conductor loop (21, 22, 28) which is interrupted by at least one capacitor (23). The sensitivity of the antenna (100) is formed by overlapping sensitivity profiles of the stripline resonator (10) and the conductor loop arrangement (20). Also described are an antenna array (200) including a plurality of antennas (100), a magnetic resonance device (300) including at least one antenna (100) or antenna array (200), and methods for magnetic resonance imaging or magnetic resonance spectroscopy.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
Magnetic resonance spectrum reconstruction method based on block Hankel matrix
InactiveCN105808869AComplex mathematical operationsMagnetic resonance spectroscopicUltrasound attenuation
The invention discloses a magnetic resonance spectrum reconstruction method based on a block Hankel matrix. The method comprises the following steps: firstly acquiring a magnetic resonance signal under-sampling template, constructing a block Hankel matrix of a two-dimensional matrix, and establishing a low-rank reconstruction model based on the block Hankel matrix of a magnetic resonance free attenuation signal; and then solving the reconstruction model through an iteration algorithm and acquiring a reconstructed free attenuation signal; and finally performing the Fourier transform to obtain a complete magnetic resonance spectrum. The reconstruction method disclosed by the invention is small in required sampling data amount, high in precision, and capable of reconstructing a complete magnetic resonance spectrum from less under-sampling data; the sampling speed of the method provided by the invention is faster than that of the existing method under the condition of obtaining the reconstructed spectrum data with the same quality.
Owner:XIAMEN UNIV OF TECH
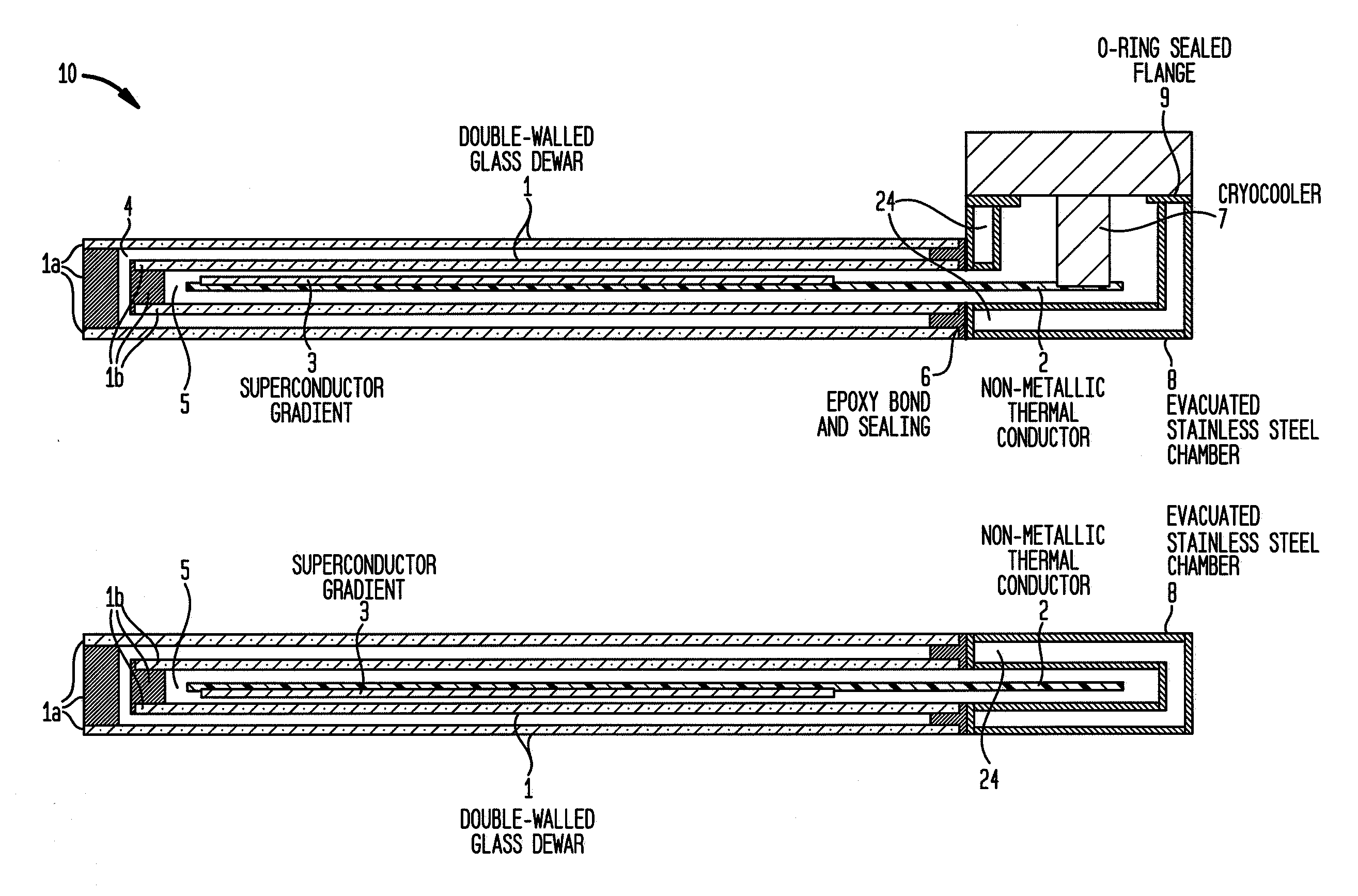
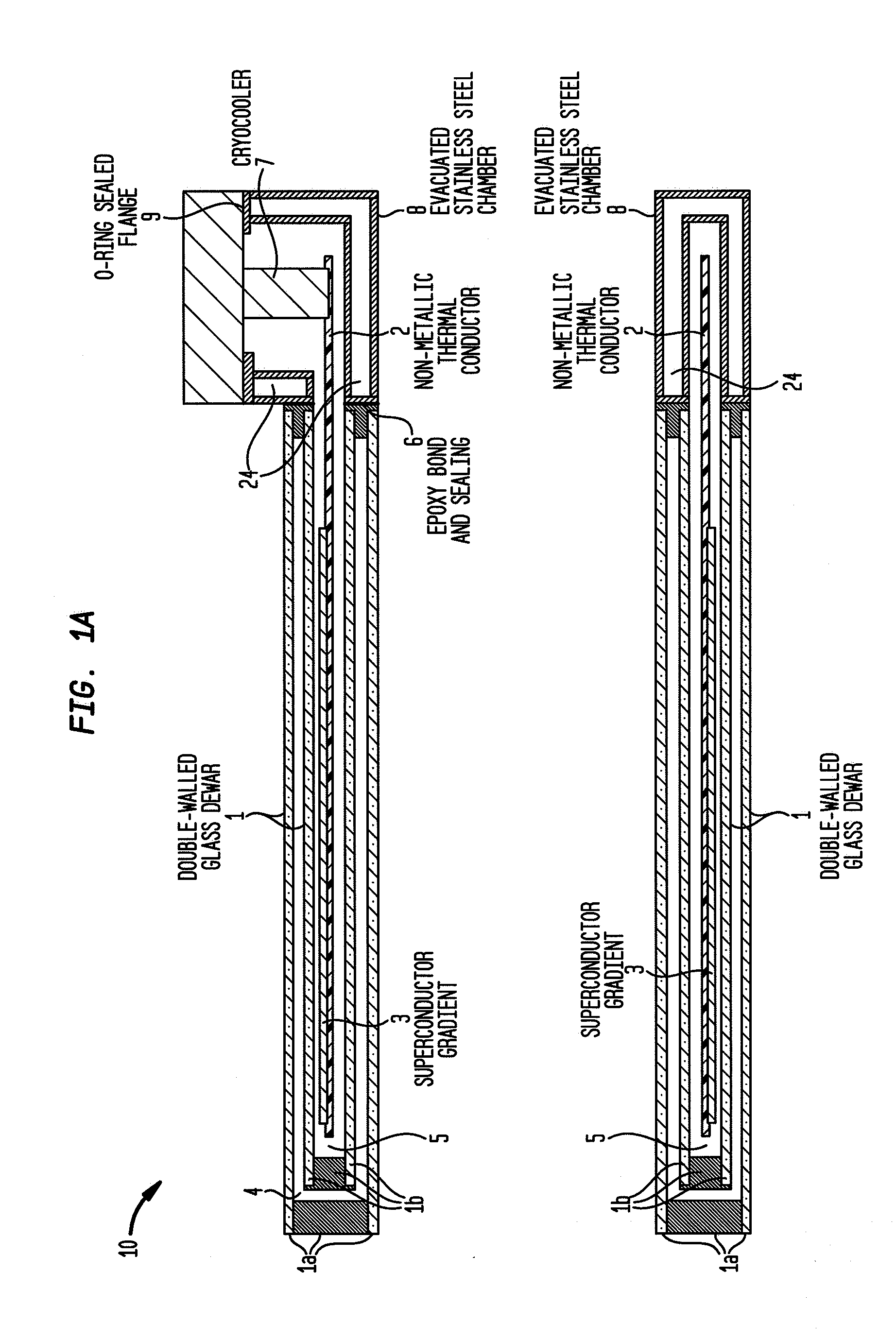
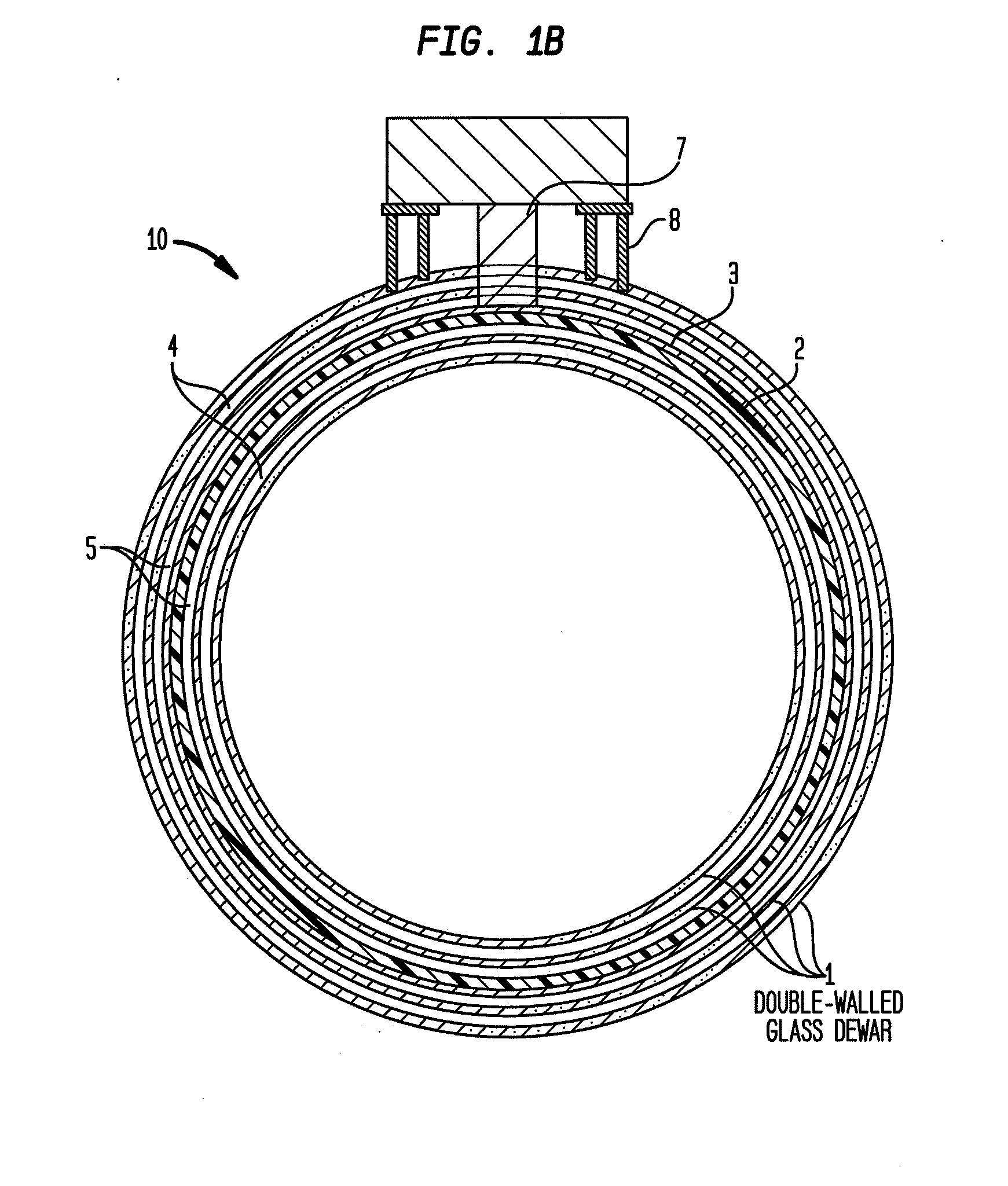
Cryogenically cooled superconductor gradient coil module for magnetic resonance imaging
ActiveUS20110012599A1Electric/magnetic detectionMeasurements using NMRThermal isolationInterior space
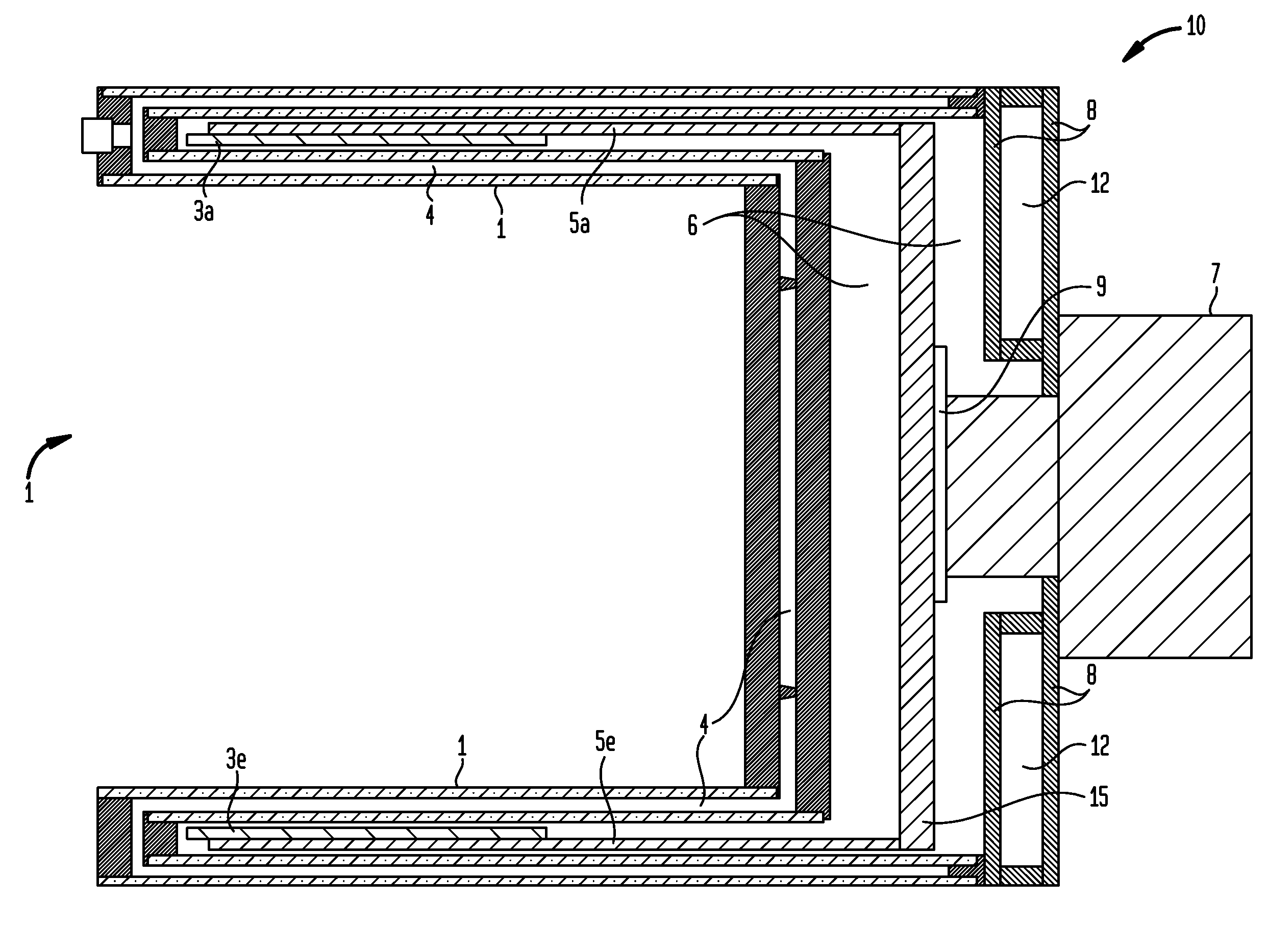
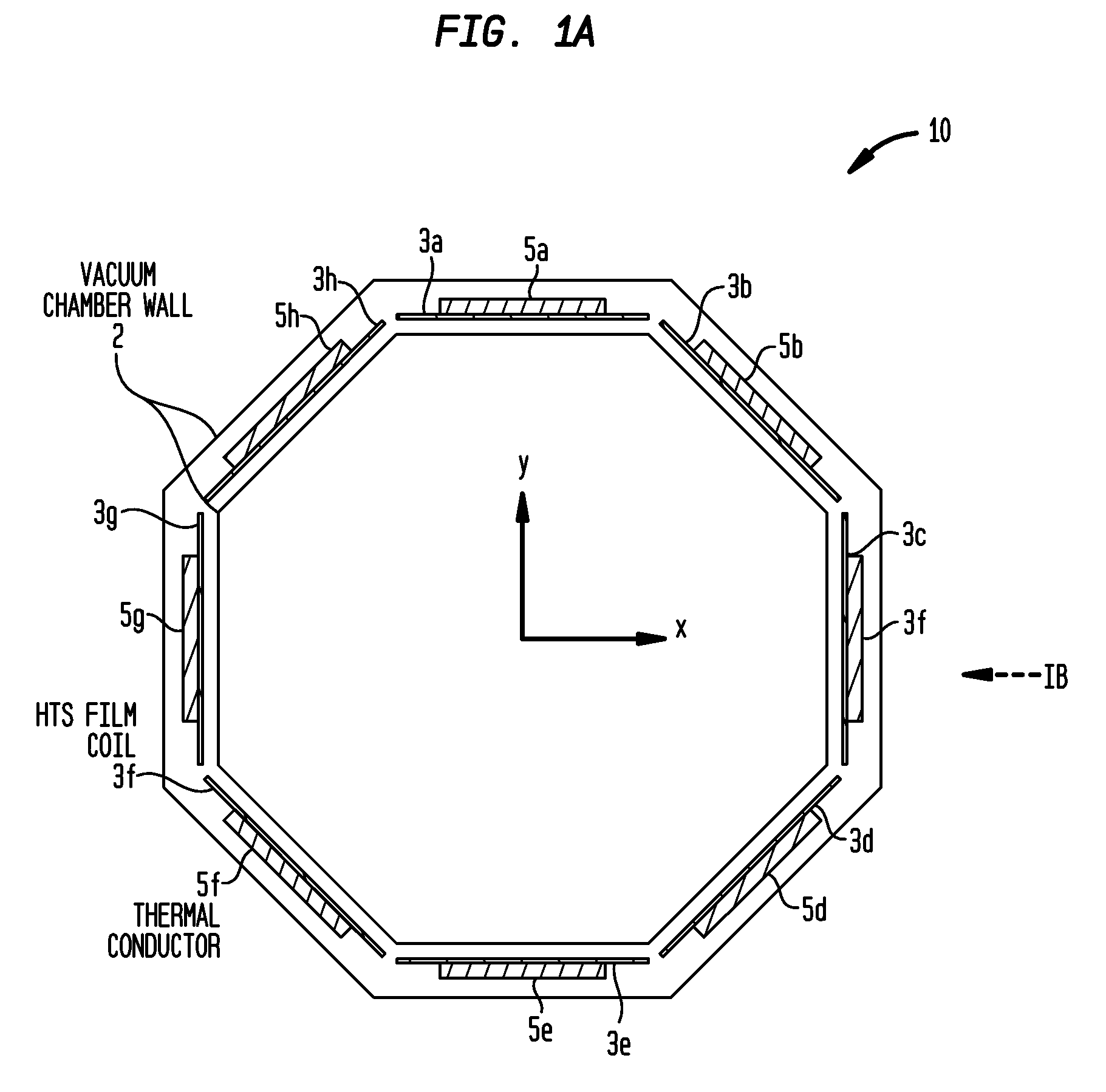
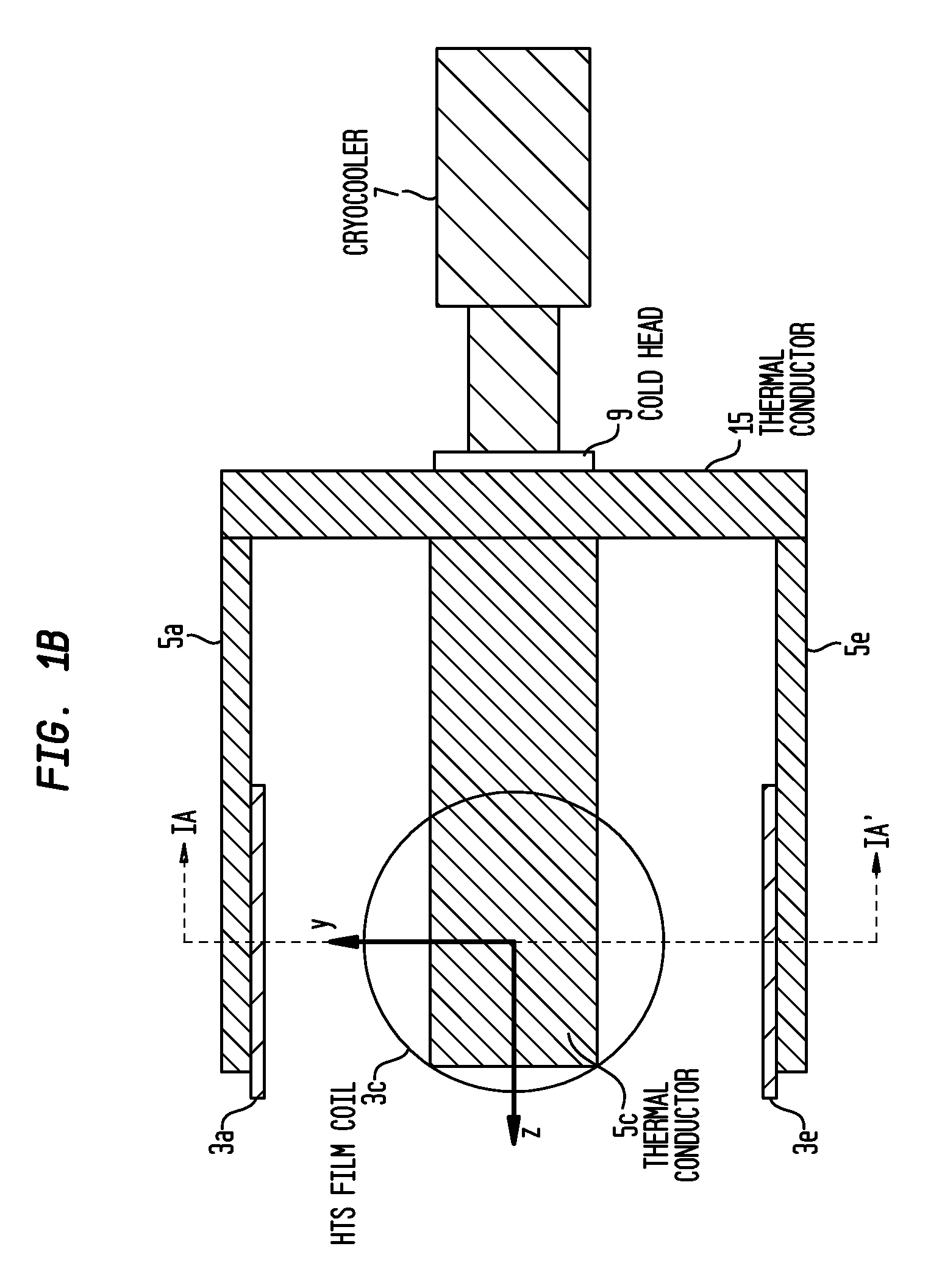
Methods and / or apparatuses for magnetic resonance imaging (MRI) and / or magnetic resonance spectroscopy comprising a superconducting gradient coil module configured for cryogenic cooling. Such a superconducting gradient coil module may comprise a vacuum thermal isolation housing comprising a double wall hermetically sealed jacket that (i) encloses a hermetically sealed interior space having a first vacuum pressure, and (ii) substantially encloses a vacuum space having a second vacuum pressure; at least one superconductor gradient coil disposed in the vacuum space; a thermal sink member disposed in the vacuum space and in thermal contact with the at least one superconductor gradient coil; and a port configured for cryogenically cooling at least the thermal sink member.
Owner:TIME MEDICAL HLDG
A magnetic resonance spectrum reconstruction method based on deep learning
ActiveCN109903259ARebuild fastHigh quality reconstructed spectraImage enhancementMagnetic measurementsTime domainMagnetic resonance spectroscopic
The invention discloses a magnetic resonance spectrum reconstruction method based on deep learning, and relates to a magnetic resonance spectrum reconstruction method. The method includes: Generatinga time domain signal of the magnetic resonance spectrum by utilizing an exponential function; Establishing a training set of the under-sampling time domain signals and the full-sampling spectrum; Designing a convolutional neural network in the data verification convolutional neural network structure; Designing a bottleneck layer in the data verification convolutional neural network structure; Designing a data verification layer in the data verification convolutional neural network structure; Designing a feedback function in a data verification convolutional neural network structure; Establishing a data verification convolutional neural network structure as a spectrum reconstruction model; Training network optimization parameters; Reconstructing an under-sampling magnetic resonance time domain signal of the target; When undersampling operation is carried out in the time-frequency domain, utilizing the strong fitting capability of the convolutional neural network and the data verification capability of the data verification layer to complete rapid and high-quality reconstruction of the undersampling magnetic resonance spectrum signal.
Owner:XIAMEN UNIV
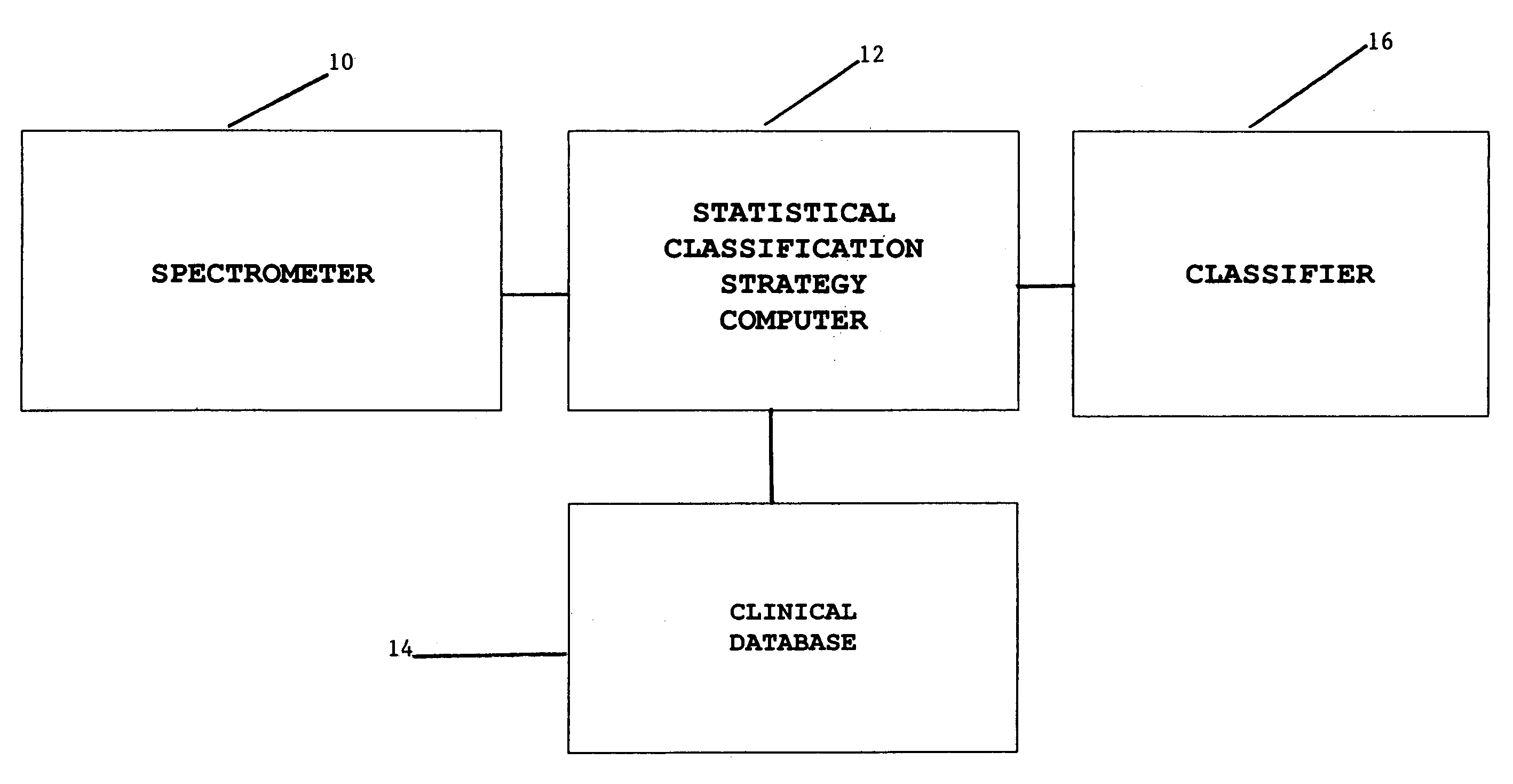
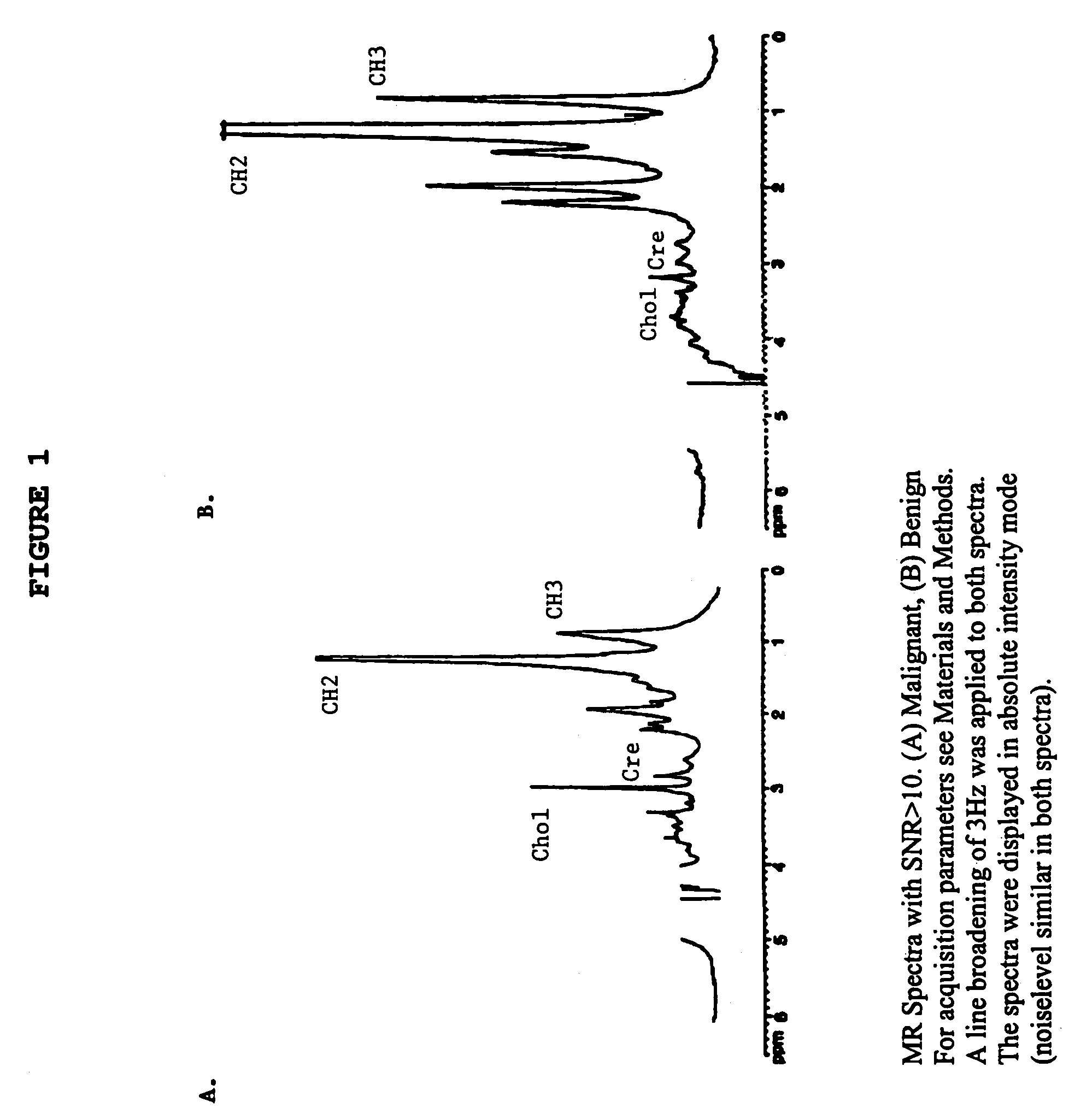
Magnetic resonance spectroscopy to classify tissue
InactiveUS7335511B2Reliable determinationComputer-assisted medical data acquisitionMeasurements using NMR spectroscopyClassification methodsTumor vessel
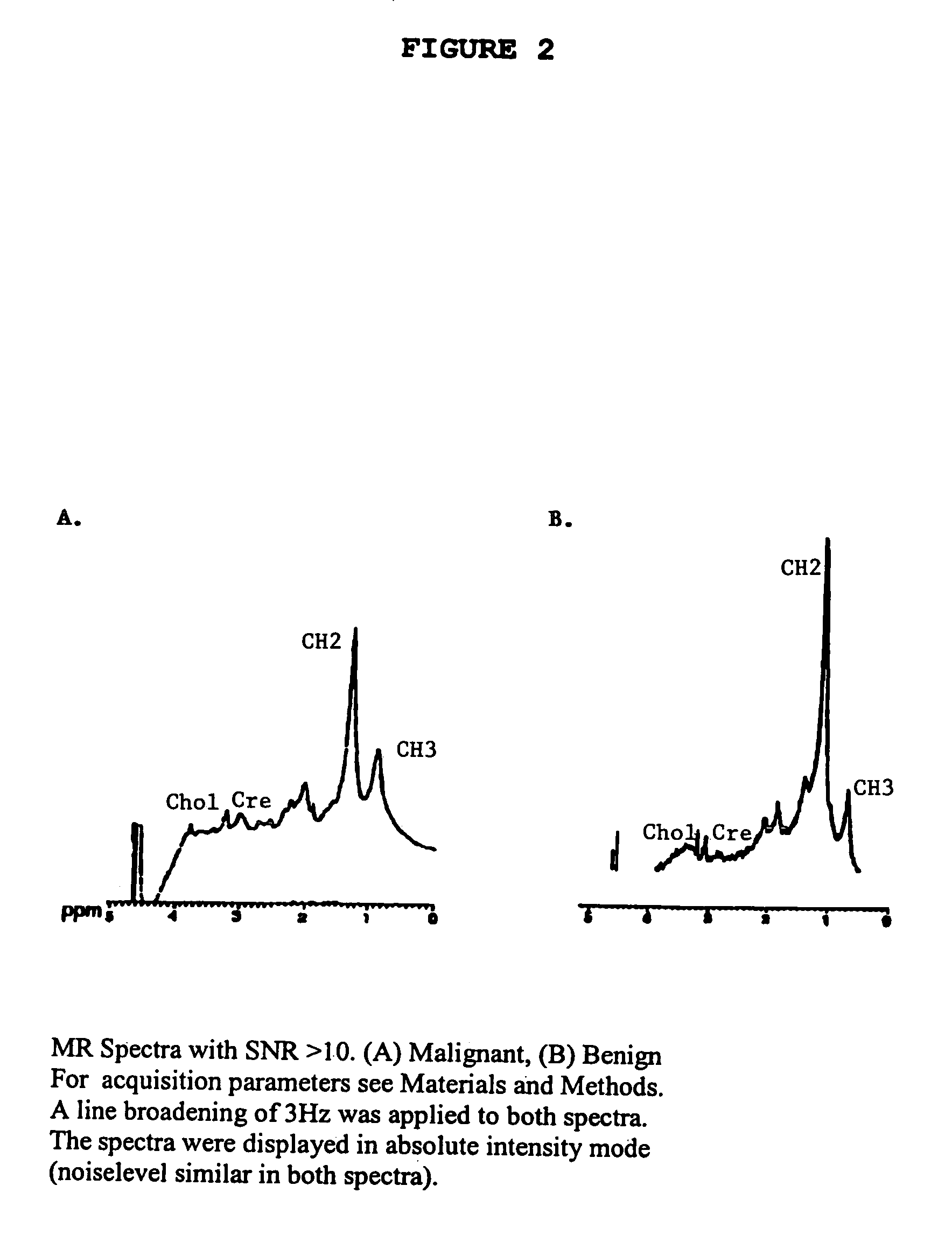
Robust classification methods analyze magnetic resonance spectroscopy (MRS) data (spectra) of fine needle aspirates taken from breast tumors. The resultant data when compared with the histopathology and clinical criteria provide computerized classification-based diagnosis and prognosis with a very high degree of accuracy and reliability. Diagnostic correlation performed between the spectra and standard synoptic pathology findings contain detail regarding the pathology (malignant versus benign), vascular invasion by the primary cancer and lymph node involvement of the excised axillary lymph nodes. The classification strategy consisted of three stages: pre-processing of MR magnitude spectra to identify optimal spectral regions, cross-validated Linear Discriminant Analysis, and classification aggregation via Computerised Consensus Diagnosis. Malignant tissue was distinguished from benign lesions with an overall accuracy of 93%. From the same spectrum, lymph node involvement was predicted with an accuracy of 95% and tumor vascularisation with an overall accuracy of 92%.
Owner:SYDNEY UNIV OF
Cryogenically cooled superconductor RF head coil array and head-only magnetic resonance imaging (MRI) system using same
A cryogenically-cooled superconducting RF head-coil array which may be used in whole-body MRI scanners and / or in dedicated, head-only MRI systems. An RF head-coil array module may comprise a vacuum thermal isolation housing comprising a double wall hermetically sealed jacket that (i) encloses a hermetically sealed interior space under a vacuum condition, and (ii) substantially encloses an interior chamber region that is separate from the hermetically sealed interior space and is configured to be evacuated to a vacuum condition. A plurality of superconductor radiofrequency coils are disposed in the interior chamber region, and each radiofrequency coil is configured for at least one of generating and receiving a radiofrequency signal for at least one of magnetic resonance imaging and magnetic resonance spectroscopy. At least one thermal sink member may be disposed in the interior chamber region and in thermal contact with the superconductor radiofrequency coils. A port is configured for cryogenically cooling the thermal sink members.
Owner:TIME MEDICAL HLDG
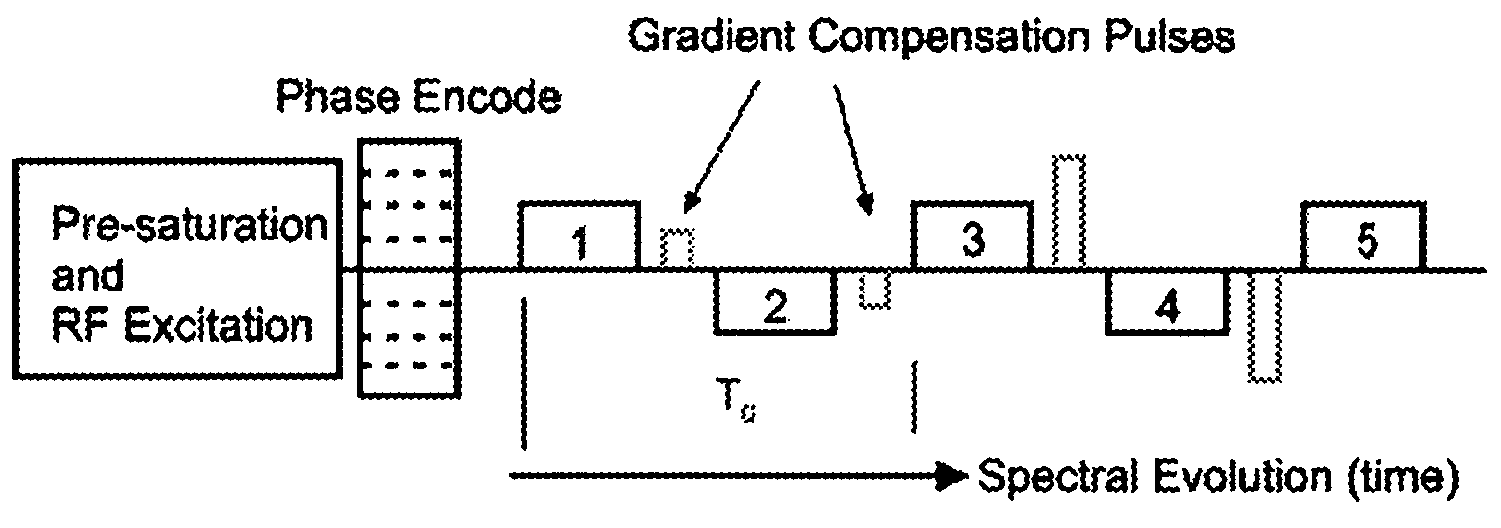
Method and MR apparatus for dynamic frequency detection in MR spectroscopy
InactiveUS7215119B2Easy to correctEasy diagnosisAnalysis using nuclear magnetic resonanceMeasurements using NMR imaging systemsMagnetic resonance spectroscopicFrequency spectrum
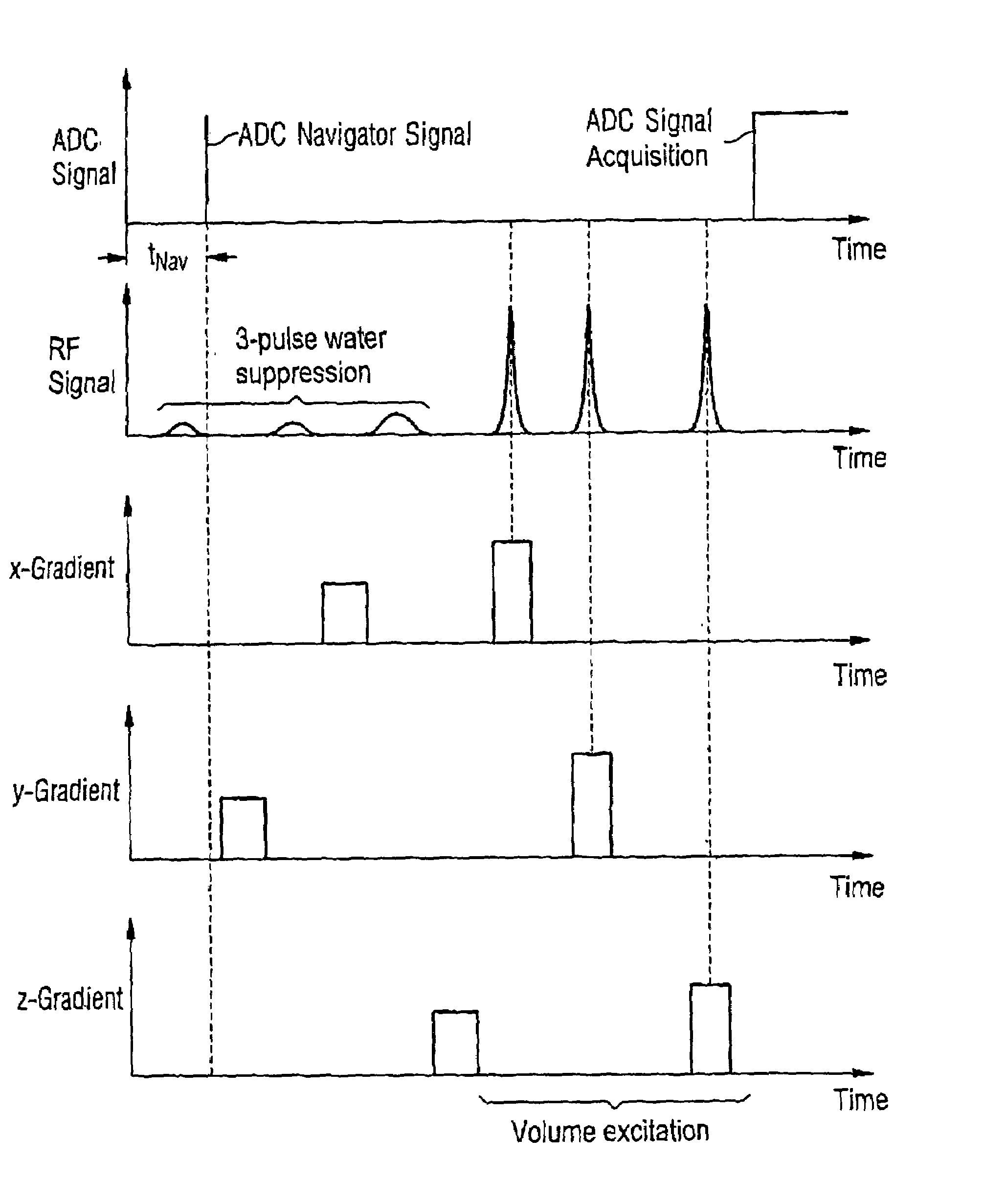
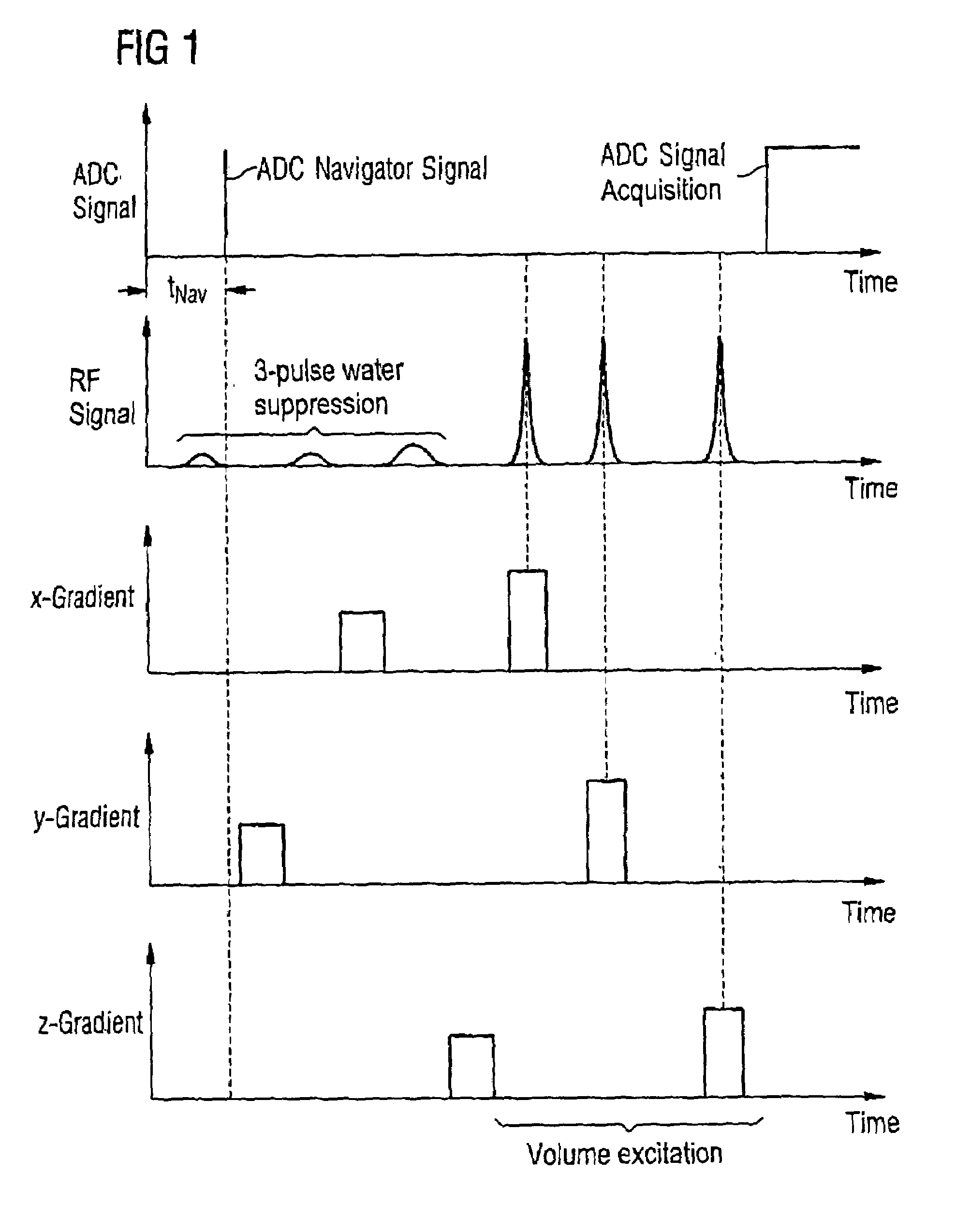
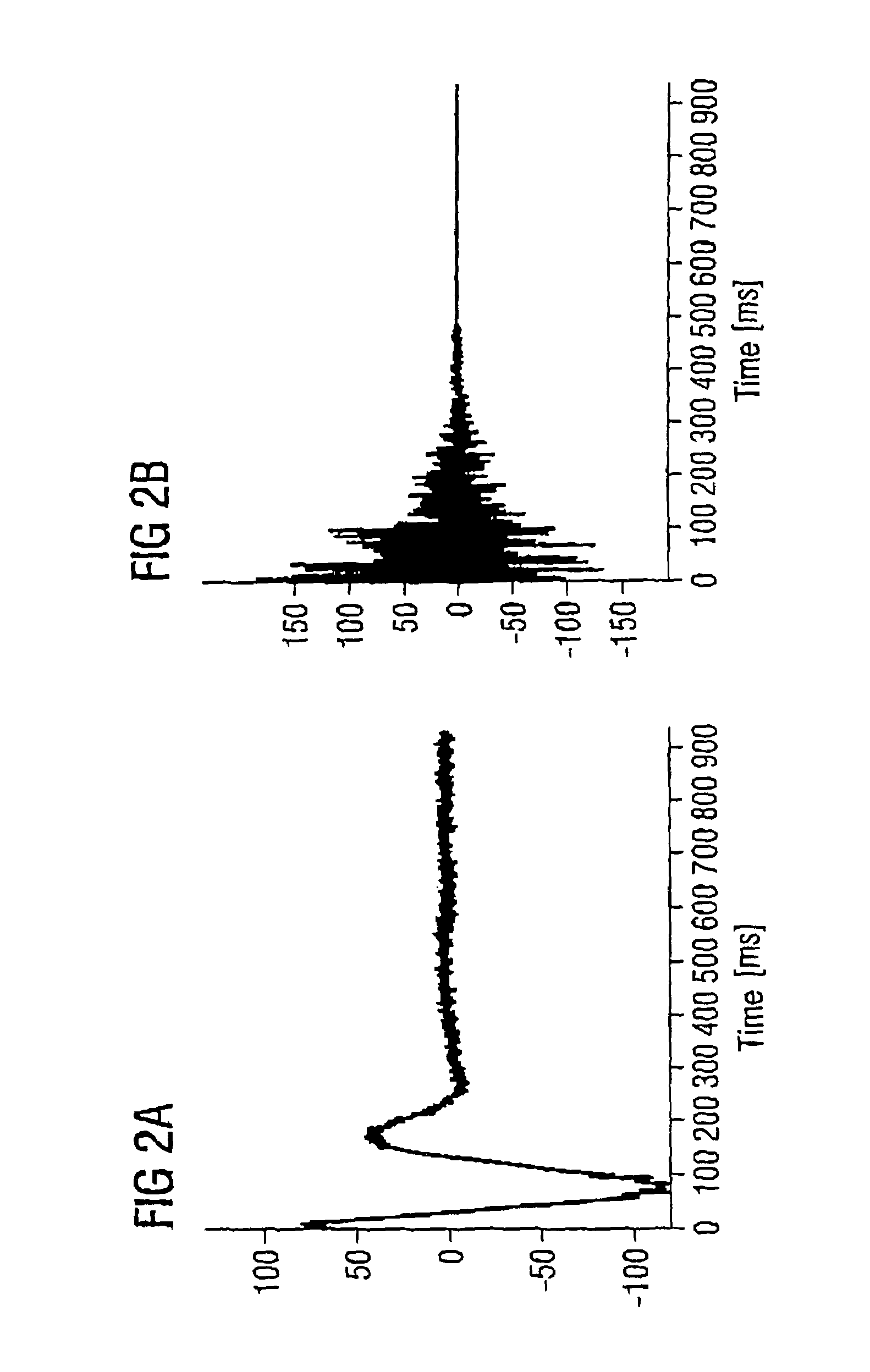
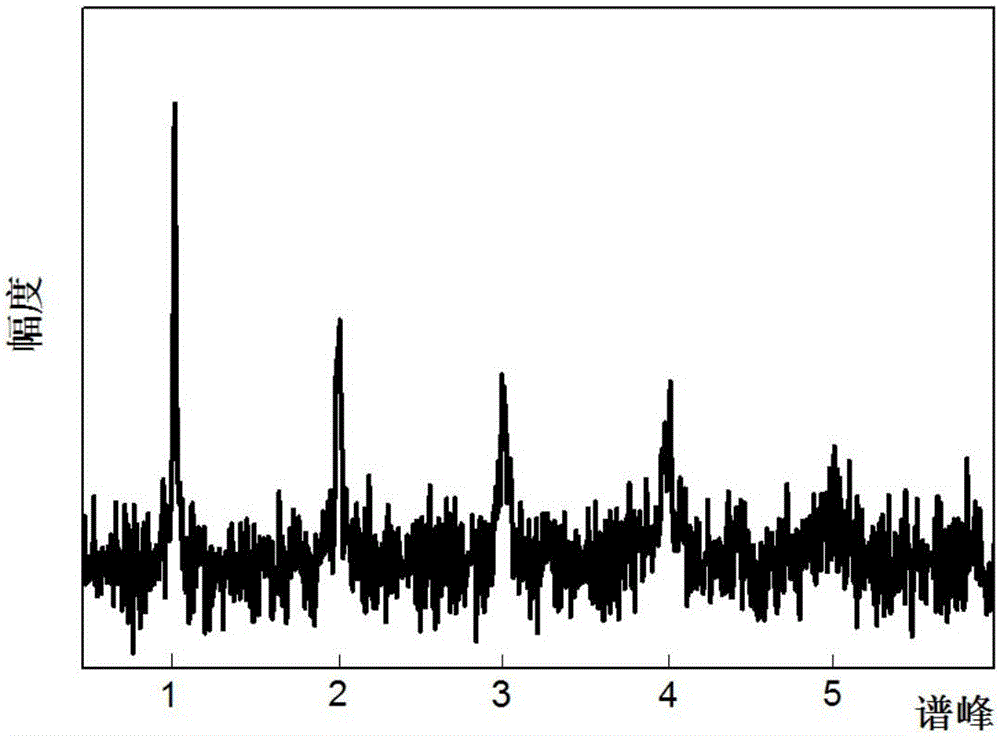
In a method for dynamic frequency detection of the resonance frequency in magnetic resonance spectroscopy, by measuring navigator signals at the same point in time in each of a number of successive sequence passes, and by comparing the navigator signals, a frequency shift of the resonance frequency is determined on the basis of which the respective individual spectrum obtained from each sequence repetition is corrected with respect to the measured frequency shift.
Owner:SIEMENS HEALTHCARE GMBH
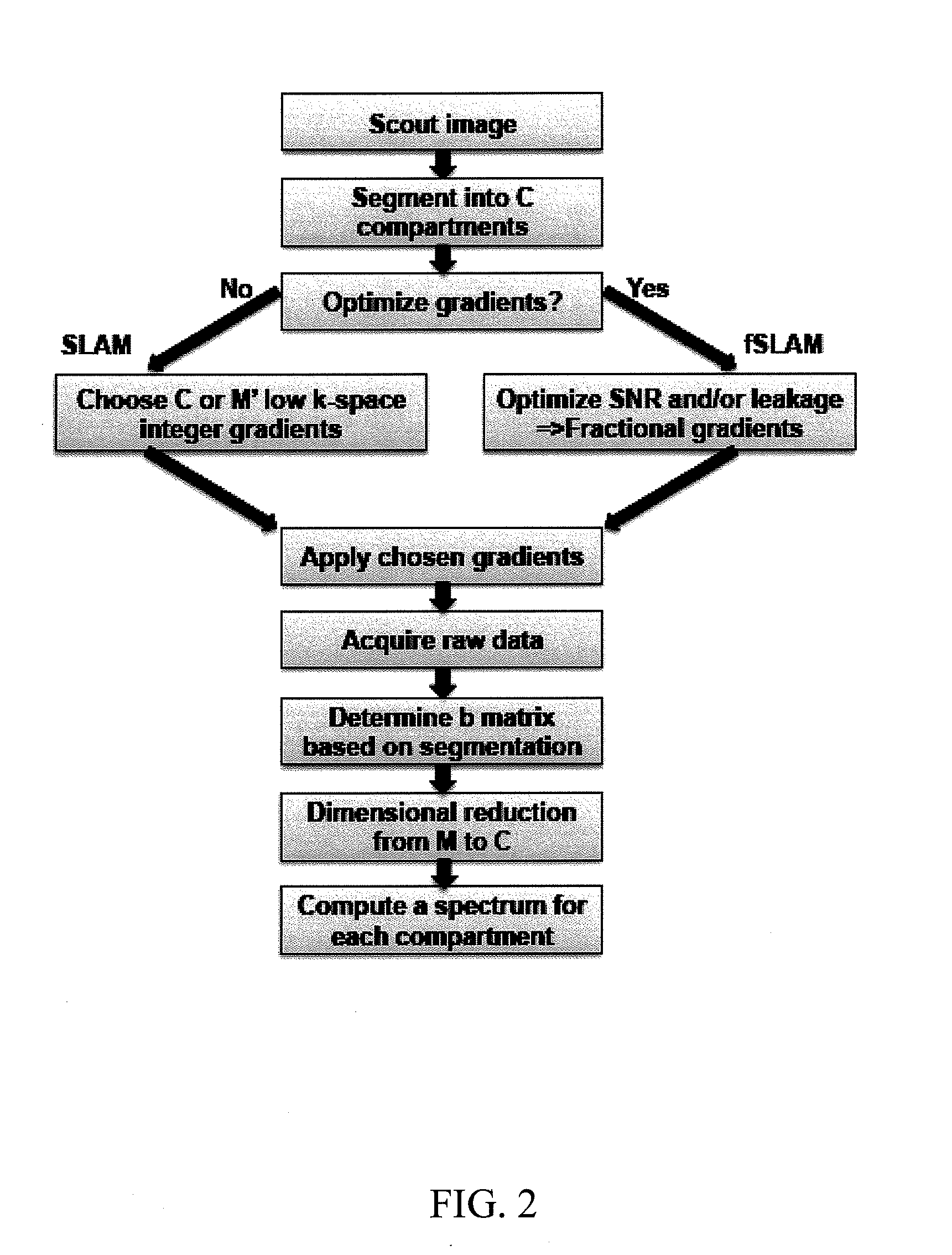
System and method of performing magnetic resonance spectroscopic imaging
ActiveUS20140015529A1Measurements using NMR spectroscopyDiagnostic recording/measuringMagnetic resonance spectroscopicSpatial average
A method of performing spatially localized magnetic resonance spectroscopy includes receiving a magnetic resonance image of an object; identifying a plurality C of compartments that generate magnetic resonance spectroscopy signals in the object including at least one compartment of interest; segmenting in at least one spatial dimension the magnetic resonance image of the object into the C compartments; acquiring magnetic resonance spectroscopy signals from the compartments by applying a plurality of M′ phase encodings applied in the at least one spatial dimension, wherein M′≧C; calculating a spatially localized magnetic resonance chemical shift spectrum from the at least one compartment of interest; and rendering a spatially localized magnetic resonance spectrum that is substantially equal to a spatial average of magnetic resonance chemical shift spectra from the at least one compartment of interest. A magnetic resonance spectroscopy and imaging system is configured to perform the above method.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Exponential signal denoising method achieved by means of prior information
ActiveCN105807241ATo achieve the purpose of denoisingReduce acquisition timeMeasurements using NMR spectroscopyMagnetic variable regulationSingular value decompositionMagnetic resonance spectroscopic
The invention provides an exponential signal denoising method achieved by means of prior information and relates to a denoising method for exponential signals.The prior exponential signals form a Hankel matrix according to a set sequence, the Hankel matrix is subjected to singular value decomposition, and a prior signal space and a prior singular value are obtained; then a Hankel matrix with the same size as the former Hankel matrix is established for the target exponential signals, and the matrix of the target signals is decomposed through the prior information space; after a weighted threshold is obtained from the prior singular value, a Hankel matrix of the denoised target signals is obtained from the singular value of the target exponential signals according to the weighted threshold; then the Hankel matrix of the target signals is solved, and finally the denoised signals are obtained.Through the prior information of the reference signals, the speed is high, the effect is good, and operation is easy.Denoising of a magnetic resonance spectrum can be achieved for signals with exponential features such as time domain signals of the magnetic resonance spectrum through the method, and the purposes of shortening the sampling time and increasing the signal to noise ratio of the spectrum are achieved.
Owner:XIAMEN UNIV
A control apparatus for controlling a therapeutic apparatus
InactiveCN102405078AUltrasound therapyMagnetic measurementsMagnetic resonance spectroscopicImaging processing
A control apparatus (106) for controlling a therapeutic apparatus (100), wherein the control apparatus comprises: -an ultrasound control interface (110) for controlling a therapeutic ultrasound system (102), -a magnetic resonance control interface (112) for controlling a magnetic resonance apparatus (104) adapted for acquiring magnetic resonance imaging data from a subject and for acquiring magnetic resonance spectroscopy data from a subject (244), -an image processing module (124, 126, 128) for generating at least one magnetic resonance imaging image (500) from the magnetic resonance imaging data and for generating at least one magnetic resonance spectroscopy map (502, 514, 516, 518, 520) from the magnetic resonance spectroscopy data, -a planning module (120); adapted for receiving the magnetic resonance imaging image and the magnetic resonance spectroscopy map and for outputting planning data (732), -a control module (122) adapted for controlling the therapeutic ultrasound system using the ultrasound control apparatus using the planning data, wherein the control module is further adapted for controlling the acquisition of the acquiring magnetic resonance imaging data and magnetic resonance spectroscopy data using the magnetic resonance control interface.
Owner:KONINK PHILIPS ELECTRONICS NV
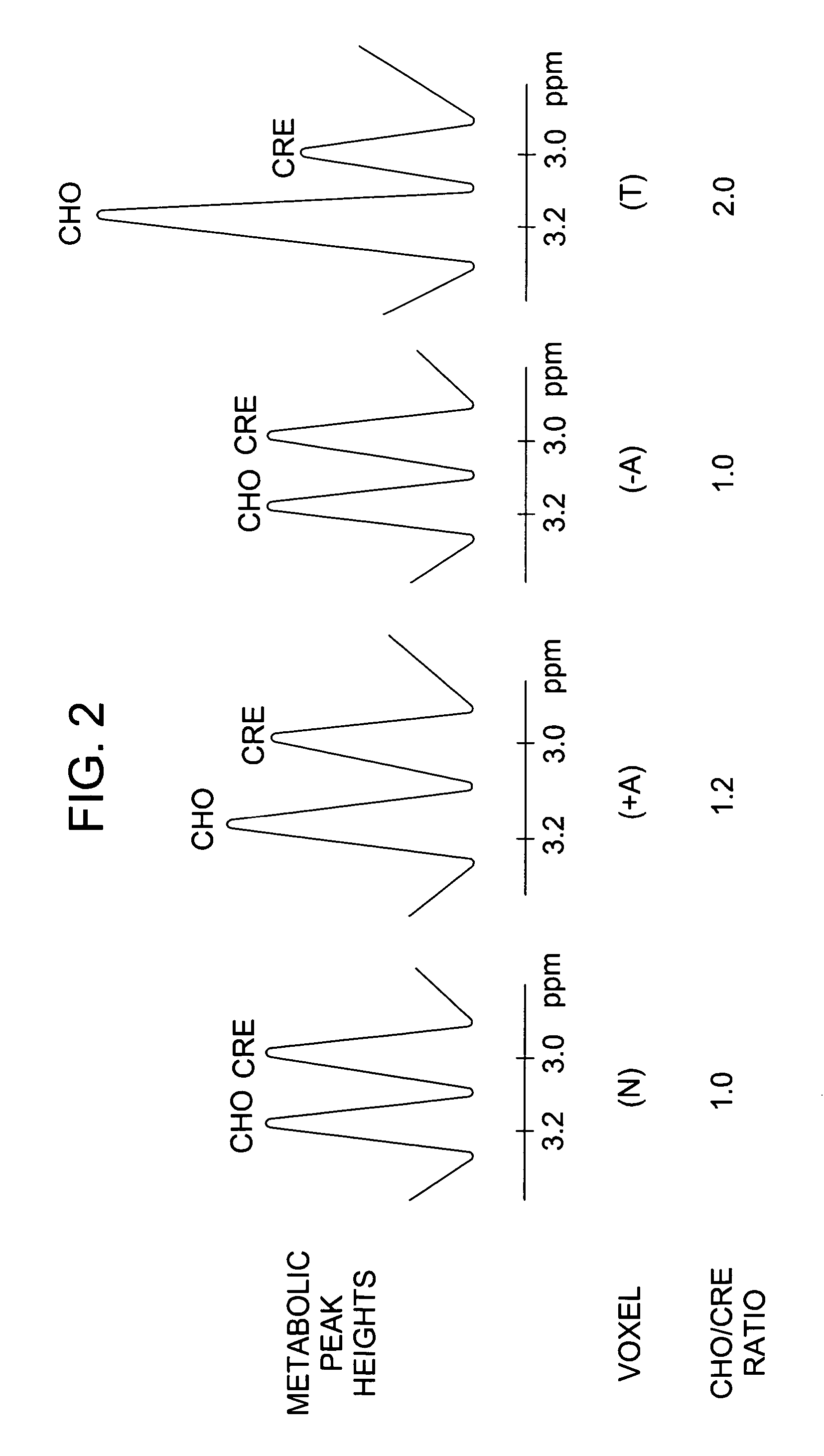
Method for monitoring early treatment response
InactiveUS20060177377A1Monitor early treatment responseCompounds screening/testingIn-vivo radioactive preparationsDiseaseCell membrane
Disclosed is a method for monitoring early treatment response of a cancer treatment comprising measuring by magnetic resonance spectroscopy (MRS), for example, proton MRS, the amount of Choline present in the tissue adjoining or surrounding the cancerous tissue before and after treatment; the treatment comprises administration of an angiogenesis inhibitor, for example, a VEGF inhibitor, whereby a decrease in the amount of Choline after treatment is indicative of a positive response. The decrease in the amount of Choline represents the decrease in the internal cell membrane as a result of down regulation of the organelles and their secretory granules and their transport vesicles. Disclosed also is a method for determining effectiveness of an angiogenesis inhibitor in the treatment of cancer. Also disclosed are methods of monitoring early treatment response in diseases where an angiogenesis effector, i.e., an inhibitor or promoter of angiogenesis, is employed.
Owner:RECEPTOMON LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com