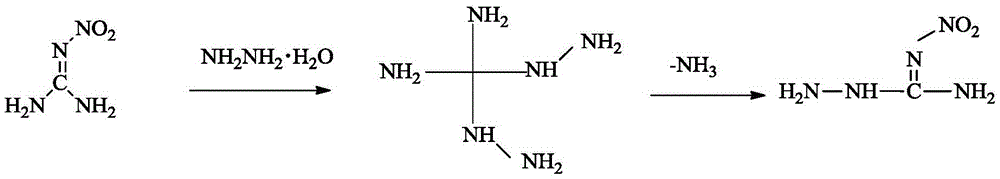
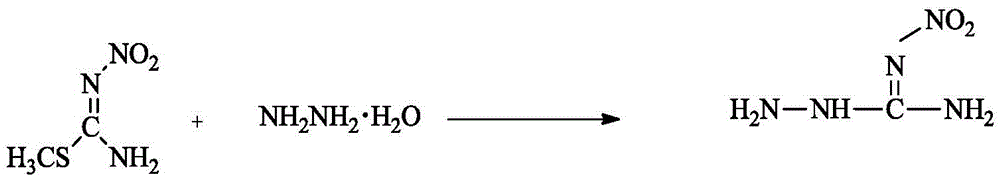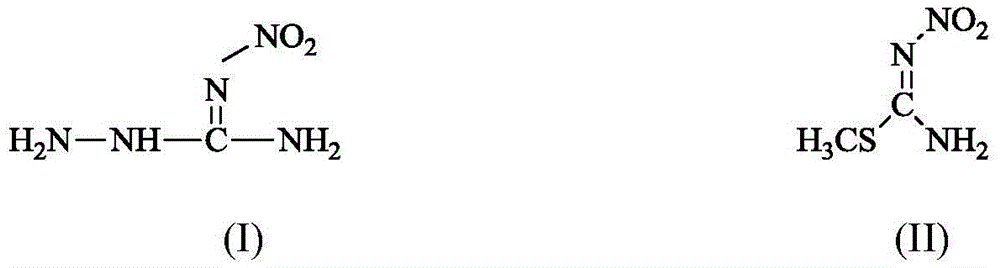Method for synthesizing 1-amino-3-nitroguanidine
A synthesis method and technology of nitroguanidine, which is applied in the field of synthesis of 1-amino-3-nitroguanidine, can solve the problems of complex processing and long reaction steps, and achieve the effect of simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] At room temperature, add 2g (19.4mmol) 2-methyl-3-nitroisothiourea into 40mL of water, add 80% 1.21g (19.4mmol) hydrazine hydrate dropwise, raise the temperature to 50°C after adding, keep it warm for 5h, and cool , filtered, and the filter cake was rinsed with ethanol and dried to obtain 1.28 g of a white solid, with a yield of 50%.
[0019] Structure Identification
[0020] Infrared Spectrum IR(KBr,cm -1 )ν: 3390, 3323, 3295, 3216, 1668, 1618, 1578, 1281, 1179, 1010, 708, 639
[0021] NMR spectrum: 13 CNMR (DMSO-d6, 125MHz), δ: 161.549;
[0022] 1 HNMR (DMSO-d 6 ,500MHz) δ:9.324,8.267,7.549,4.678
[0023] Elemental Analysis: Structural Formula C 1 h 5 N 5 o 2
[0024] Theoretical value: C10.08, H4.20, N58.82;
[0025] Measured value: C9.83, H4.35, N58.73;
[0026] The above structure identification data confirm that the substance obtained in this step is indeed 1-amino-3-nitroguanidine.
Embodiment 2
[0028] At room temperature, add 4g (38.8mmol) 2-methyl-3-nitroisothiourea into 40mL of water, add 80% 1.21g (19.4mmol) hydrazine hydrate dropwise, raise the temperature to 50°C after adding, keep it warm for 5h, and cool , filtered, and the filter cake was rinsed with ethanol and dried to obtain 1.15 g of a white solid, with a yield of 45%.
Embodiment 3
[0030] At room temperature, add 2g (19.4mmol) 2-methyl-3-nitroisothiourea into 40mL of water, add 80% 1.21g (19.4mmol) hydrazine hydrate dropwise, raise the temperature to 70°C after adding, keep it warm for 6h, and cool , filtered, and the filter cake was rinsed with ethanol and dried to obtain 1.2 g of a white solid, with a yield of 47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com