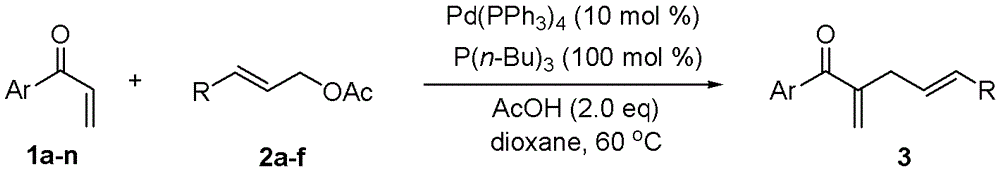MBH reaction of alpha, beta-unsaturated ketone and allyl acetate
An allyl acetate, unsaturated technology, applied in the separation/purification of carbonyl compounds, preparation of carbonyl compounds, chemical instruments and methods, etc., to achieve the effects of high yield, easier preparation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, utilize α, the MBH reaction method of β-unsaturated ketone and allyl acetate, carry out following steps successively:
[0029] 1), in α, β-unsaturated ketone (0.1mmol) and acetic acid (12.0mg, 0.2mmol) in 1,4-dioxane solution (2mL), add tributylphosphine P (n-Bu) 3 (20.2 mg, 0.1 mmol). After the mixture was stirred for 1 minute, Pd(PPh 3 ) 4 (11.6 mg, 0.01 mmol) and allyl acetate (0.3 mmol). The mixture was stirred and reacted at 60° C. for 24 hours.
[0030] The α,β-unsaturated ketone is in particular 1a p-methylphenyl vinyl ketone;
[0031] Allyl acetate is specifically 2a allyl acetate;
[0032] 2), the reaction product obtained in step 1) was rotary evaporated to remove the solvent (i.e., 1,4-dioxane), and the residue was purified by column chromatography, using 200-300 mesh silica gel, and ethyl acetate:petroleum ether =1:100 (volume ratio) as eluent, and the obtained eluate was rotary evaporated to obtain 2-methylene-1-(p-tolyl)pent-4-en-1-one(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com