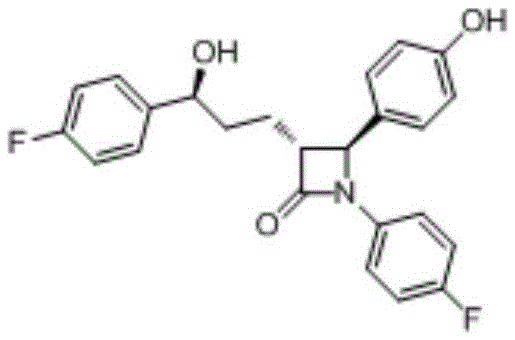
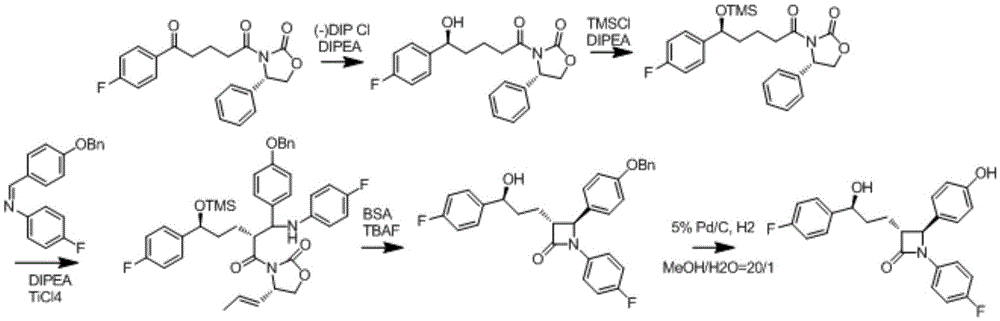
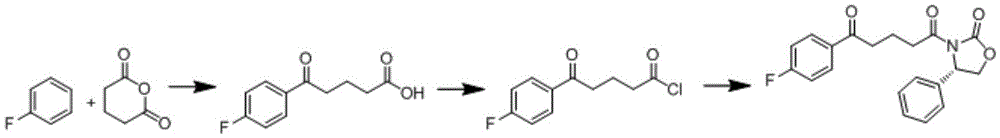
Preparation method of ezetimibe intermediate
A technology of ezetimibe and an intermediate, which is applied in the field of drug synthesis, can solve problems such as troublesome reaction products, and achieve the effects of short reaction route, high yield and few separation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of ((1-(4-fluorophenyl)vinyl)oxy)trimethylsilane
[0037] The structural formula is as follows:
[0038]
[0039] In a 500ml three-necked flask, add 20g (0.145mol, 1eq.) of 1-(4-fluorophenyl) ethyl ketone, 22g (0.217mol, 1.5eq) of triethylamine, and 18.90g (0.174mol, 1.2eq) and 100ml of N,N-dimethylformamide, the reaction was stirred under the protection of nitrogen, the temperature was raised to 100°C for 10 hours, and then cooled to room temperature (25°C), the reaction solution was diluted with 150ml of petroleum ether, and the precipitate precipitated The triethylamine hydrochloride was removed by suction filtration with a Buchner funnel, the filtrate was collected, and the organic phase was washed with 200 ml of ice 10% (wt) sodium bicarbonate solution, and the organic phase was washed twice with 200 ml of water. The solvent was distilled off under reduced pressure. , 27.1 g of solid was obtained, with a yield of 90%.
Embodiment 2
[0040] Example 2 Preparation of ((1-(4-fluorophenyl)vinyl)oxy)trimethylsilane
[0041] The structural formula is as follows:
[0042]
[0043] In a 500ml three-necked flask, add 20g (0.145mol, 1eq.) of 1-(4-fluorophenyl)ethanone, 26.18g (0.203mol, 1.4eq) of diisopropylethylamine, and 19.69g of trimethylchlorosilane (0.181mol, 1.25eq) and 100ml of toluene, the reaction was stirred under nitrogen protection, the temperature was raised to 100°C for 8 hours, then cooled to room temperature, the reaction solution was diluted with 150ml petroleum ether, and the precipitated triethylamine hydrochloride was used The Buchner funnel was removed by suction, the filtrate was collected, and the organic phase was washed with 200 ml of ice 10% (wt) sodium bicarbonate solution. The organic phase was washed twice with 200 ml of water. The solvent was distilled off under reduced pressure to obtain 25.59 g of solid. Rate 85%).
Embodiment 3
[0044] Example 3 Preparation of ((1-(4-fluorophenyl)vinyl)oxy)trimethylsilane
[0045] The structural formula is as follows:
[0046]
[0047] In a 500ml three-necked flask, add 20g (0.145mol, 1eq.) of 1-(4-fluorophenyl)ethanone, 26.18g (0.203mol, 1.4eq) of diisopropylethylamine, and 27.70g of bromotrimethylsilane (0.181mol, 1.25eq) and 100ml of toluene, the reaction was stirred under nitrogen protection, the temperature was raised to 100°C for 12 hours, then cooled to room temperature, the reaction solution was diluted with 150ml petroleum ether, and the precipitated triethylamine hydrochloride was used The Buchner funnel was suction filtered to remove, the filtrate was collected, and the organic phase was washed with 200 ml ice 10% (wt) sodium bicarbonate solution, and the organic phase was washed twice with 200 ml water. The solvent was distilled off under reduced pressure to obtain 26.50 g of solid. The rate is 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com