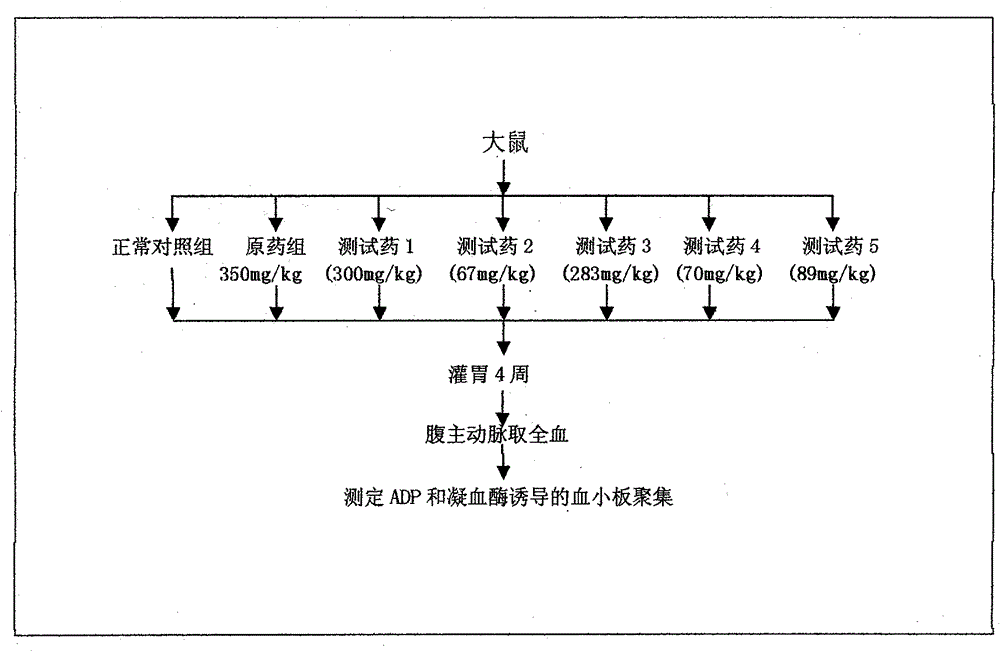
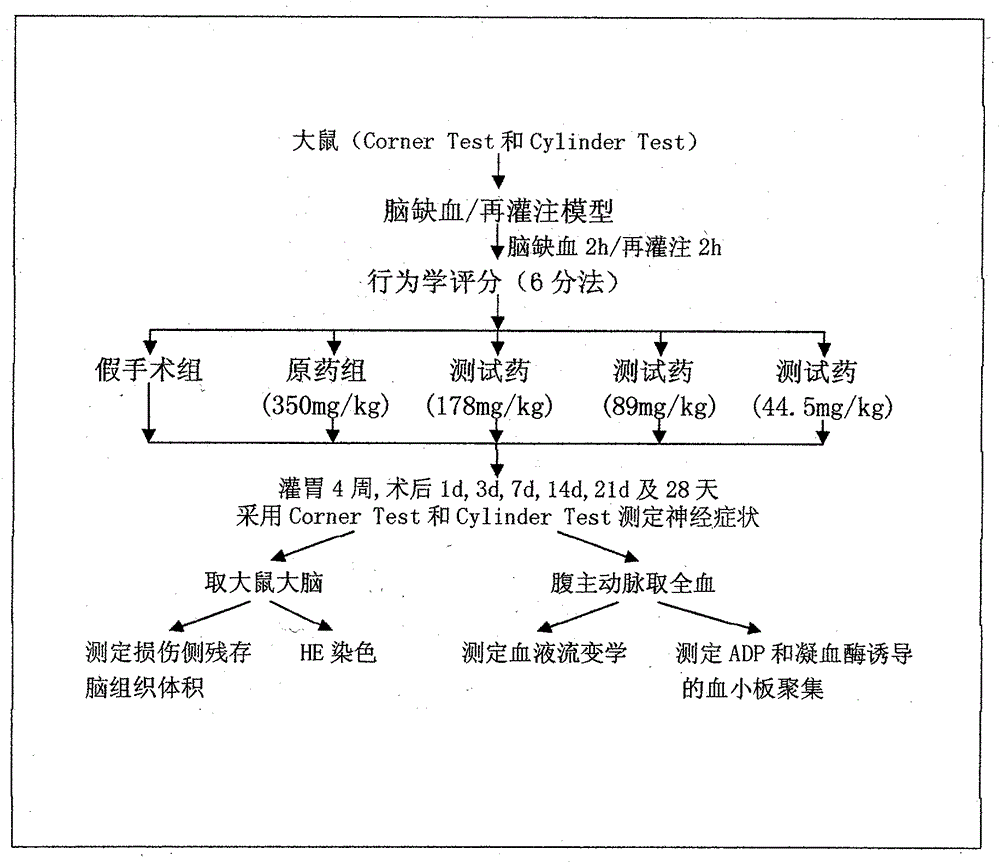
Cerebrovascular disease prevention and treatment pharmaceutical preparation and preparation method thereof
A technology for cerebrovascular diseases and pharmaceutical preparations, which is applied in the field of pharmaceutical preparations for the prevention and treatment of cerebrovascular diseases and its preparation, and can solve the problems of poorly reflecting the quality and curative effect of preparations, rough preparation process, and inconvenient medication.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
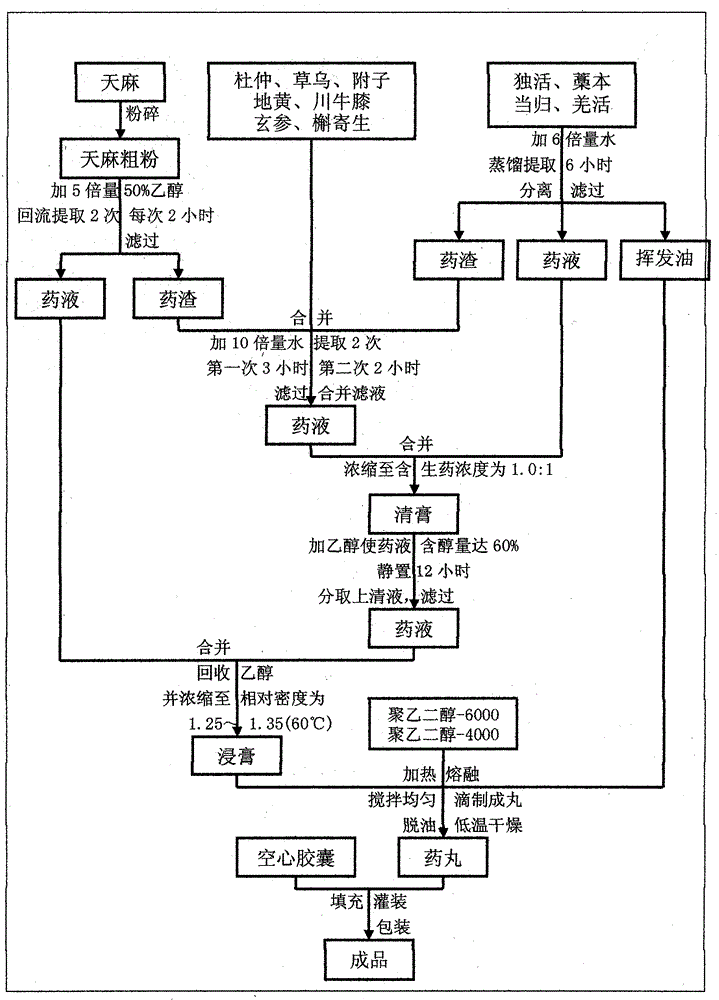
[0272] Example 1: 139.2g Gastrodia elata, 147.8g Eucommia ulmoides (made from salt), 17.4g made Aconitum aconitum, 17.4g aconite (made), 86.8g Duhuo, 102.6g Ligusticum edulis, 102.6g Scrophulariaceae, 174g angelica, 278.2g rehmannia glutinosa, Sichuan Achyranthes bidentata 102.6g, mistletoe 102.6g, notopterygium 174g.
[0273] Weigh the Gastrodia elata raw material, add 5 times the amount of 50% ethanol to reflux and extract 3 times, each time for 2 hours, combine, filter, and obtain the ethanol extract for later use, and the remaining Gastrodia elata medicine residue for later use; The four raw materials are steam distilled and extracted for 6 hours, separated, and the volatile oil is obtained for later use; Double the amount of water to extract twice, the first time for 3 hours, the second time for 2 hours, combine the extracts, filter, the filtrate is concentrated to a medicinal solution containing crude drug concentration of 1:1, let cool to room temperature, add ethanol t...
Embodiment 2
[0274] Example 2: Gastrodia elata 1392g, Eucommia ulmoides (salt) 1478g, Aconitum ulmoides 174g, Fuzi (made) 174g, Duhuo 868g, Ligusticum 1026g, Scrophulariaceae 1026g, Angelica 1740g, Rehmannia glutinosa 2782g, Achyranthes bidentata 1026g, Mistletoe 1026g , Notopterygium 1740g.
[0275] Weigh the Gastrodia elata raw material, add 5 times the amount of 50% ethanol to reflux and extract 3 times, each time for 2 hours, combine, filter, and obtain the ethanol extract for later use, and the remaining Gastrodia elata medicine residue for later use; The four raw materials are steam distilled and extracted for 6 hours, separated, and the volatile oil is obtained for later use; Double the amount of water to extract twice, the first time for 3 hours, the second time for 2 hours, combine the extracts, filter, the filtrate is concentrated to a medicinal solution containing crude drug concentration of 1:1, let cool to room temperature, add ethanol to make the solution contain The alcohol...
Embodiment 3
[0276] Example 3: 139.2g Gastrodia elata, 147.8g Eucommia ulmoides (made from salt), 17.4g made Aconitum aconitum, 17.4g Fuzi (made), 86.8g Duhuo, 102.6g Ligusticum edulis, 102.6g Scrophulariaceae, 174g Angelica, 278.2g Rehmannia glutinosa, Sichuan Achyranthes bidentata 102.6g, mistletoe 102.6g, notopterygium 174g.
[0277]Weigh the raw medicinal materials of Gastrodia elata, add 3 times the amount of 90% ethanol to reflux and extract once, extract for 4 hours, combine, filter, and obtain the ethanol extract for later use, and the remaining Gastrodia elata medicine residue for later use; Flavored raw medicinal materials were extracted by steam distillation for 10 hours, separated, and volatile oil was obtained for later use; the remaining medicinal residues were combined with Gastrodia elata medicinal residues, Eucommia ulmoides, Aconitum aconitum, Aconite, Scrophulariaceae, Rehmannia glutinosa, Achyranthes bidentata, and mistletoe, and added 12 times Measure water and extract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com