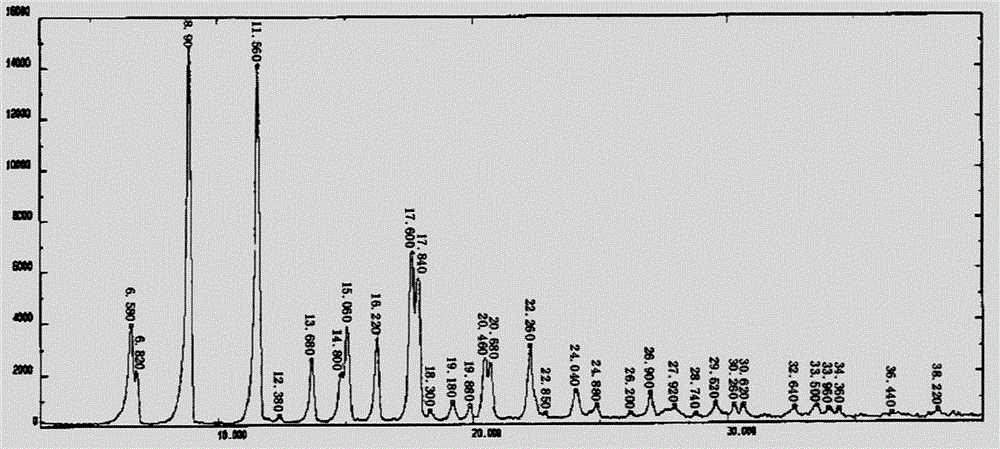
Preparation method of difluprednate beta form crystal
A technology of difluprednate and crystal form, which is applied in the field of preparation of difluprednate crystal form β, can solve problems such as crystal form instability, and achieve the effects of stable impurities, high yield, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 10 g of difluprednate and 80 ml of absolute ethanol to the reaction flask, heat to 75-80° C. to dissolve, and filter. Transfer the filtrate and the precipitated solids to a one-necked bottle, heat in a water bath at 60°C and concentrate by rotary evaporation until the remaining volume is about 50ml, then reheat to reflux to dissolve. Cool down and crystallize at a rate of 0.5°C / min. Stir at -10°C for 30 minutes, filter, and dry in the air to obtain the crystal form β of difluprednate. Yield 92%, purity 99%.
Embodiment 2
[0023] Add 10 g of difluprednate and 40 ml of absolute ethanol to the reaction flask, heat to 75-80° C. to dissolve, and filter. Transfer the filtrate and the precipitated solids to a one-necked bottle, heat in a water bath at 55°C and concentrate by rotary evaporation until the remaining volume is about 45ml, then reheat to reflux to dissolve. Cool down and crystallize at a rate of 0.3°C / min. Stir at -10°C for 30 minutes, filter, and dry in the air to obtain the crystal form β of difluprednate. Yield 91.5%, purity 99.1%.
Embodiment 3
[0025] Add 10 g of difluprednate and 100 ml of absolute ethanol to the reaction flask, heat to 75-80° C. to dissolve, and filter. Transfer the filtrate and the precipitated solid into a one-necked bottle, heat and concentrate in a water bath at 65°C by rotary evaporation until the remaining volume is about 55ml, and reheat to reflux to dissolve. Cool down at a rate of 2°C / min for crystallization. Stir at -10°C for 30 minutes, filter, and dry in the air to obtain the crystal form β of difluprednate. Yield 91%, purity 98.5%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com