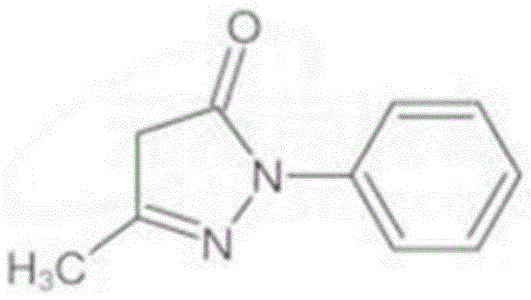
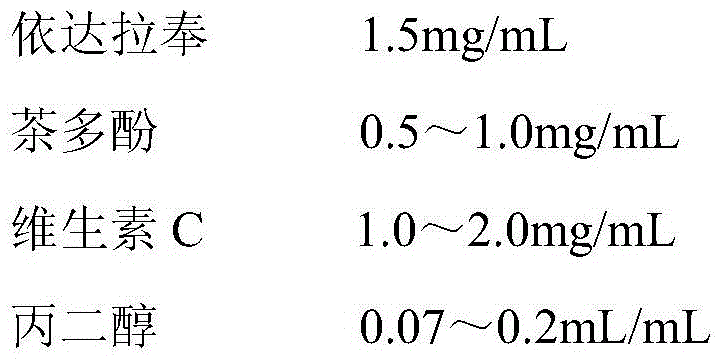Edaravone medicine composition and preparation method thereof
A technology of edaravone and composition, applied in the directions of drug combination, pharmaceutical formulation, drug delivery, etc., can solve the problem of not selecting antioxidants, etc., and achieve the effects of good drug efficacy and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] prescription:
[0049]
[0050]
[0051] Preparation Process:
[0052] (1) Under the condition of nitrogen protection, take the prescribed amount of propylene glycol and 70% to 80% of the prescribed amount of water for injection to stir and mix, heat to 60°C, and the residual oxygen content in the liquid is lower than 2ppm;
[0053] (2) Under the condition of nitrogen protection, another prescribed amount of tea polyphenols and vitamin C was added and fully dissolved with water for injection;
[0054] (3) Under the condition of nitrogen protection, slowly add the tea polyphenols and vitamin C aqueous solution of step (2) into the mixed solution of propylene glycol and water in step (1), and add the prescribed amount of Eda with an average particle diameter of 20 μm at the same time Lavone, stir well until completely dissolved;
[0055] (4) Under nitrogen protection conditions, add 0.1% active carbon for needles to the mixed solution in step (3) and stir evenly, an...
Embodiment 2
[0060] prescription:
[0061]
[0062] Preparation Process:
[0063] (1) Under nitrogen protection conditions, take the prescribed amount of propylene glycol and 70% to 80% of the prescribed amount of water for injection to stir and mix, heat to 75°C, and the residual oxygen content in the liquid is lower than 2ppm;
[0064] (2) Under the condition of nitrogen protection, another prescribed amount of tea polyphenols and vitamin C was added and fully dissolved with water for injection;
[0065] (3) Under the condition of nitrogen protection, slowly add the tea polyphenols and vitamin C aqueous solution of step (2) into the mixed solution of propylene glycol and water in step (1), and add the prescribed amount of Ida with an average particle diameter of 5 μm at the same time Lavone, stir well until completely dissolved;
[0066] (4) Under nitrogen protection conditions, add 0.2% active carbon for needles to the mixed solution in step (3) and stir evenly, and filter the char...
Embodiment 3
[0071] prescription:
[0072]
[0073] Preparation Process:
[0074] (1) Under the condition of nitrogen protection, take the prescribed amount of propylene glycol and 70% to 80% of the prescribed amount of water for injection to stir and mix, heat to 80°C, and the residual oxygen content in the liquid is lower than 2ppm;
[0075] (2) Under the condition of nitrogen protection, another prescribed amount of tea polyphenols and vitamin C was added and fully dissolved with water for injection;
[0076] (3) Under the condition of nitrogen protection, slowly add the tea polyphenols and vitamin C aqueous solution of step (2) into the mixed solution of propylene glycol and water in step (1), and add the prescribed amount of Ida with an average particle diameter of 10 μm at the same time Lavone, stir well until completely dissolved;
[0077] (4) Under nitrogen protection conditions, add 0.3% activated carbon for needles to the mixed solution in step (3) and stir evenly, and filte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com