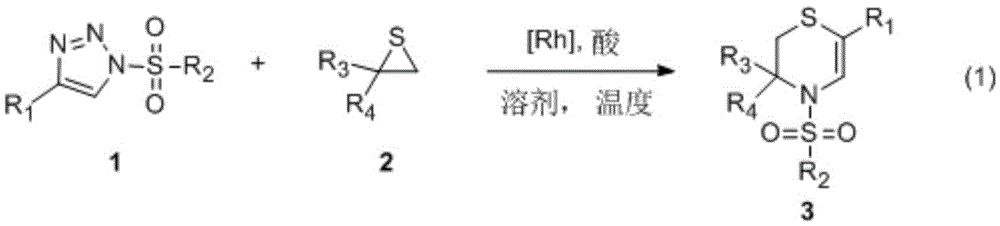
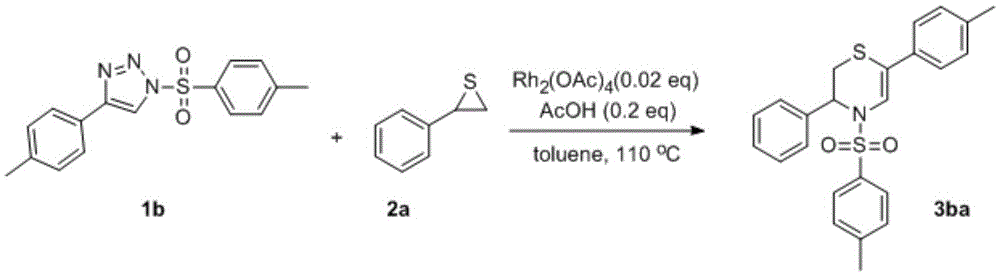
Synthetic method of N-sulfonyl-3,4-dihydro-2H-1,4-thiazine
A -2H-1, synthesis method technology, applied in the field of chemical pharmaceutical and fine chemical preparation, can solve the problems such as not found, and achieve the effect of convenient preparation, difficult preparation and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
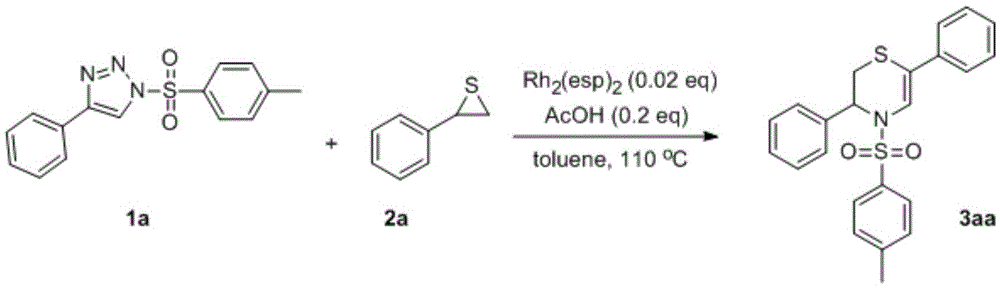
[0029] Take 10 ml of sealed tube, add 1 mmol of thiocycloethane 2a, 0.01 mmol of bis[(α,α,α',α',-tetramethyl-1,3-benzenedipropionate) rhodium], Add 0.1 mmol of acetic acid to 0.5 mmol of sulfonyltriazole 1a in 2 ml of toluene solution, evacuate the reaction solution under nitrogen protection, stir and react at 110 degrees Celsius for 1 hour, evaporate the solvent under reduced pressure, and wash the residue with ethyl acetate Using petroleum ether as eluent, silica gel column chromatography separation and purification, the corresponding N-sulfonyl-3,4-dihydro-2H-1,4-thiazine 3aa was obtained with a yield of 85%.
[0030] 3aa: white solid, mp: 137℃. 1 HNMR (300MHz, CDCl 3 )δ7.61(d, J=8.3Hz, 2H), 7.56(s, 1H), 7.49–7.46(m, 2H), 7.37–7.19(m, 10H), 5.58(t, J=3.0Hz, 1H ),2.98(dd,J=13.1,3.2Hz,1H),2.63(dd,J=13.1,3.2Hz,1H),2.40(s,3H). 13 CNMR (100MHz, CDCl 3 )δ144.2, 139.0, 137.8, 135.2, 129.9, 128.6, 128.4, 127.8, 127.7, 127.1, 126.2, 126.0, 117.6, 115.7, 54.5, 31.1, 2...
Embodiment 2
[0032] Take 10 ml of sealed tube, add 1 mmol of thiocycloethane 2a, 0.015 mmol of rhodium acetate (Rh 2 (OAc) 4 ) was added to 2.5 milliliters of 1,2-dichloroethane solution of 0.5 mmoles of sulfonyltriazole 1a, the reaction solution was evacuated under nitrogen protection, stirred and reacted for 5 hours at 80 degrees Celsius, and the solvent was evaporated under reduced pressure, and the residue The corresponding N-sulfonyl-3,4-dihydro-2H-1,4-thiazine 3aa was obtained by silica gel column chromatography using ethyl acetate and petroleum ether as eluents with a yield of 53%.
Embodiment 3
[0034] Take 10 ml sealed tube, add 0.5 mmol thiocycloethane 2a, 0.01 mmol bis[(α,α,α',α',-tetramethyl-1,3-benzenedipropionate) rhodium], Add 0.1 mmol of acetic acid to 0.5 mmol of sulfonyltriazole 1a in 1 ml of toluene solution, evacuate the reaction solution under nitrogen protection, stir and react at 110 degrees Celsius for 3 hours, evaporate the solvent under reduced pressure, and wash the residue with ethyl acetate Using petroleum ether as eluent, silica gel column chromatography separation and purification, the corresponding N-sulfonyl-3,4-dihydro-2H-1,4-thiazine 3aa was obtained with a yield of 49%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com