Novel preparation method for novel anti-hepatitis C drug-daklinza
A daclatasvir and anti-hepatitis C technology, which is applied in the field of preparation of the new anti-hepatitis C drug daclatasvir, can solve the problems of multiple synthesis steps and high economic costs, and achieve the effects of easy-to-obtain raw materials, strong applicability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
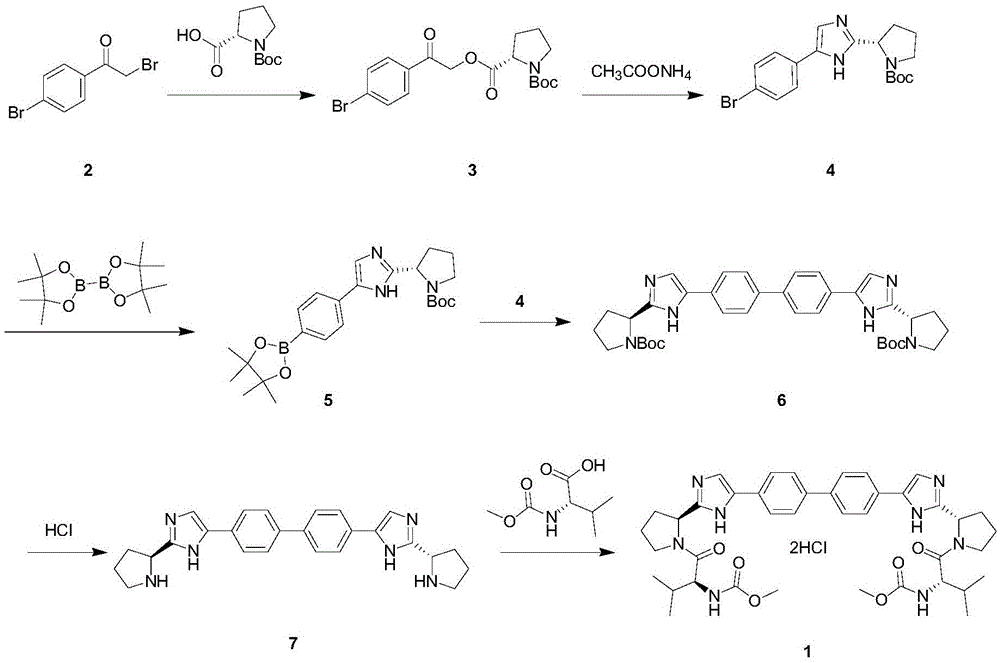
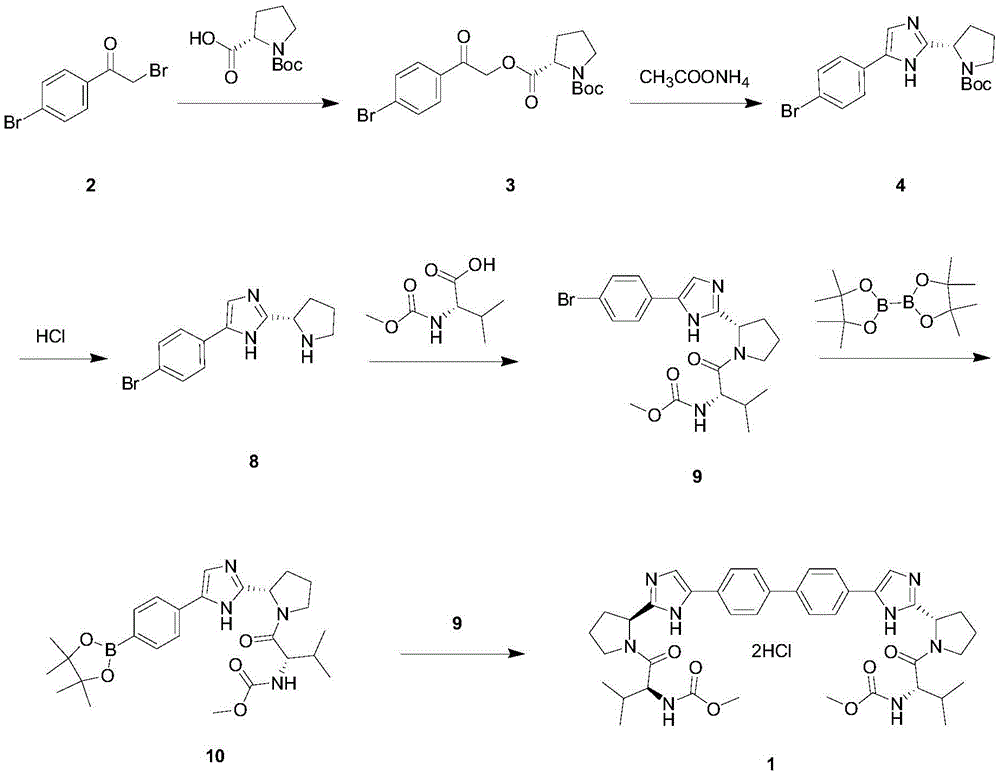
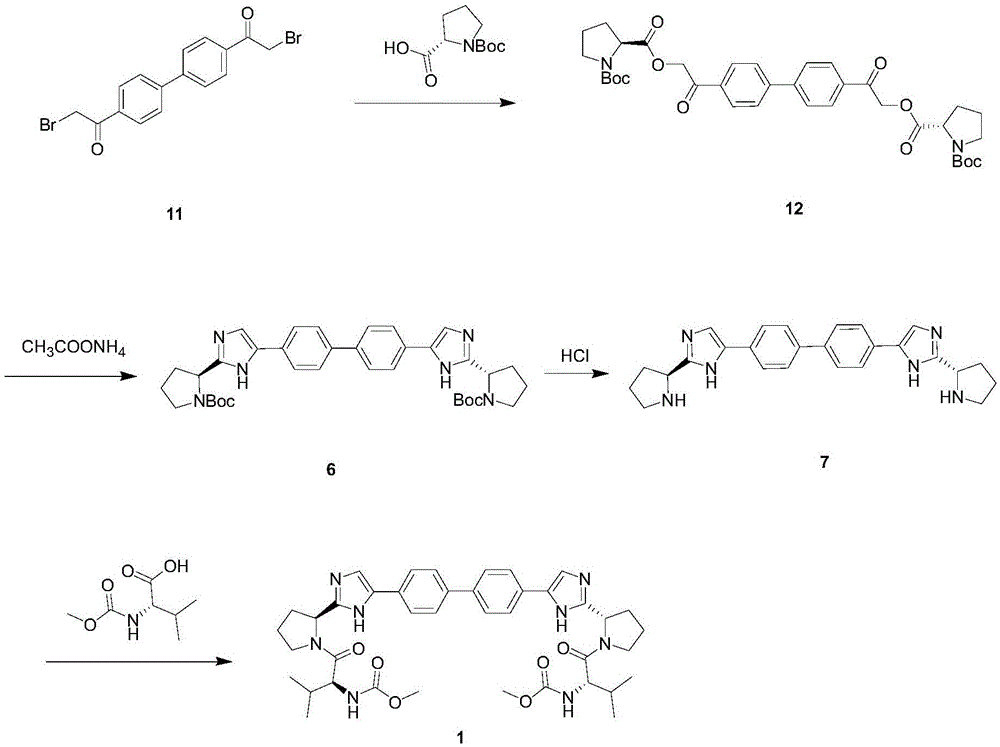
Image
Examples
Embodiment 1
[0026] Example 1 , Synthesis of ester (14)
[0027] Add 4,4'-bis(2-chloroacetyl)biphenyl (14 g, 45 mmol) and N-(methoxycarbonyl)-L-valyl-L-proline (25.7 g, 94.5 mmol) and Acetonitrile (140mL) was put in a 250mL there-necked flask, stirred until it was fully dissolved and cooled to 10°C, and diisopropylethylamine DIPEA (12.2g, 94.5mmol) was added dropwise at 10-30°C, after the addition was complete, the Stir at 40-45 degrees for 4-7 hours, the reaction is complete, the reaction solution is concentrated under reduced pressure, and replaced with toluene solution (140mL), washed twice with saturated aqueous sodium chloride solution (70mL), and the toluene is concentrated to obtain an oily foamy solid (33.7g , yield 95%).
Embodiment 2
[0028] Example 2 , the synthesis of ester (14)
[0029] Add 4,4'-bis(2-chloroacetyl)biphenyl (14 g, 45 mmol) and N-(methoxycarbonyl)-L-valyl-L-proline (25.7 g, 94.5 mmol) and Acetonitrile (140mL) was added to a 250mL three-necked flask, and diisopropylethylamine DIPEA (12.2 g, 94.5 mmol) was added dropwise at 10-15 degrees. After the addition was complete, it was stirred at 10-15 degrees for 30-36 hours, After the reaction was complete, the reaction solution was concentrated under reduced pressure and replaced with toluene solution (140 mL), washed twice with saturated chloride (70 mL), and the toluene was concentrated to obtain an oily foamy solid (32.9 g, yield 92.7%).
Embodiment 3
[0030] Example 3 , the synthesis of ester (14)
[0031] Add 4,4'-bis(2-chloroacetyl)biphenyl (14 g, 45 mmol) and N-(methoxycarbonyl)-L-valyl-L-proline (25.7 g, 94.5 mmol) and Acetonitrile (140mL) was added to a 250mL three-necked flask, and diisopropylethylamine DIPEA (12.2 g, 94.5 mmol) was added dropwise at 20-25 degrees. After the addition was complete, it was stirred at 20-25 degrees for 16-20 hours, After the reaction was complete, the reaction solution was concentrated under reduced pressure and replaced with toluene solution (140 mL), washed twice with saturated chloride (70 mL), and the toluene was concentrated to obtain an oily foamy solid (33.1 g, yield 93.2%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com