Ibrutinib amorpphis and preparation method thereof
A technology of ibrutinib and amorphous substances, which is applied in the field of medicine, can solve the problems of poor reproducibility and stability, and achieve the effect of simple operation and stable stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
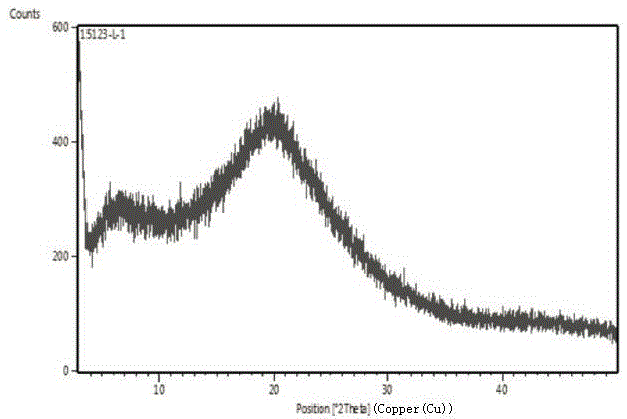
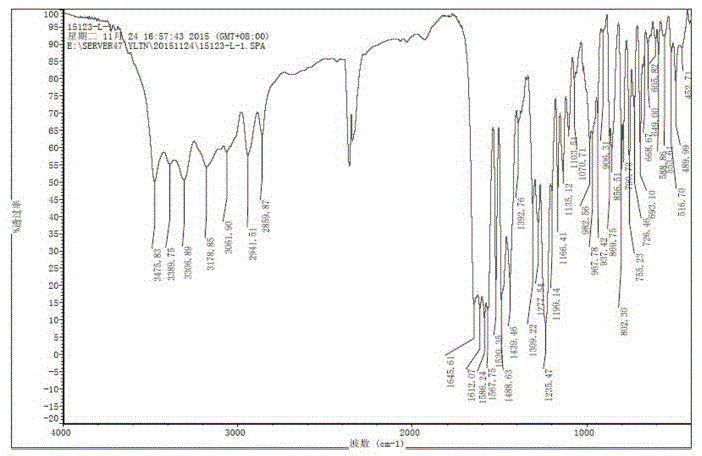
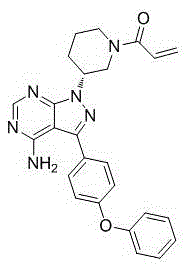
[0042] Add 1.0 g of ibrutinib into 6.0 ml of methanol, heat to 50°C, and stir to dissolve. Add the above solution to 18g of ice-water mixture, control the temperature below 5°C, stir and solidify for 30 minutes, filter, and dry the filter cake at 50°C under reduced pressure to constant weight to obtain 0.96g of white solid, HPLC: 99.57%. Test its X-ray powder diffraction, its XRPD as figure 1 ; Test infrared spectrum such as figure 2 .
Embodiment 2
[0044] Add 5.0 g of ibrutinib into 25.0 ml of ethanol, heat to 65°C, and stir to dissolve. Add the above solution to 100g of ice and 30g of ethanol mixed solution, control the temperature between -20°C and 0°C, stir and solidify for 2 hours, filter, and dry the filter cake at 70°C under reduced pressure to constant weight to obtain 4.8g of white solid , HPLC: 99.77%. The X-ray powder diffraction test is the same as the XRPD of Example 1, and the infrared spectrum of the test is basically the same as that of Example 1, that is, the amorphous ibrutinib.
Embodiment 3
[0046] Add 1.0 g of ibrutinib into 10.0 ml of acetonitrile, heat to reflux, and stir to dissolve. Add the above solution to a mixture of 18 g of ice and 3.0 g of acetone, control the temperature below 0°C, stir and solidify for 4 hours, filter, and dry the filter cake at 60°C under reduced pressure to constant weight to obtain 0.87 g of a white solid, HPLC: 99.37 %. The X-ray powder diffraction test is the same as the XRPD of Example 1, and the infrared spectrum of the test is basically the same as that of Example 1, that is, the amorphous ibrutinib.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com