Colorectal cancer detection primer, method and kit
A technology for detection kits and detection primers, applied in biochemical equipment and methods, measurement/testing of microorganisms, DNA/RNA fragments, etc., can solve the problem of invasiveness, easy complications, limited application, and inability to adapt to screening of high-risk groups and diagnosis, to achieve the effect of convenient and flexible sample collection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Down-regulation of CA4 expression in colorectal cancer:
[0041] The total cellular RNA was extracted by Trizol method and reverse-transcribed into cDNA by Applied Biosystems cDNAReverseTranscription kit, and the operations were carried out according to the method recommended by the kit. Primers CA4-F1 and CA4-R1 for semi-quantitative PCR were designed according to the human CA4 nucleotide sequence; wherein,
[0042] Primer CA4-F1: 5'-CCGGCTCAGAGGACTCTT-3',
[0043] Primer CA4-R1: 5'-GTTGGAGGACTCGGCTTGAA-3';
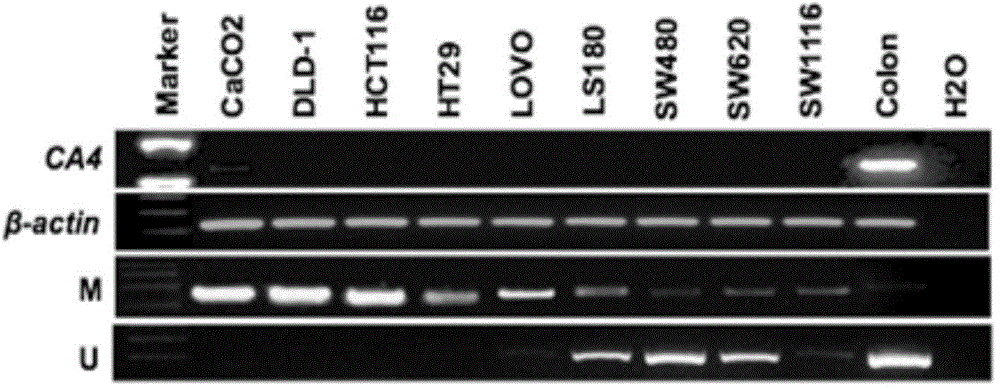
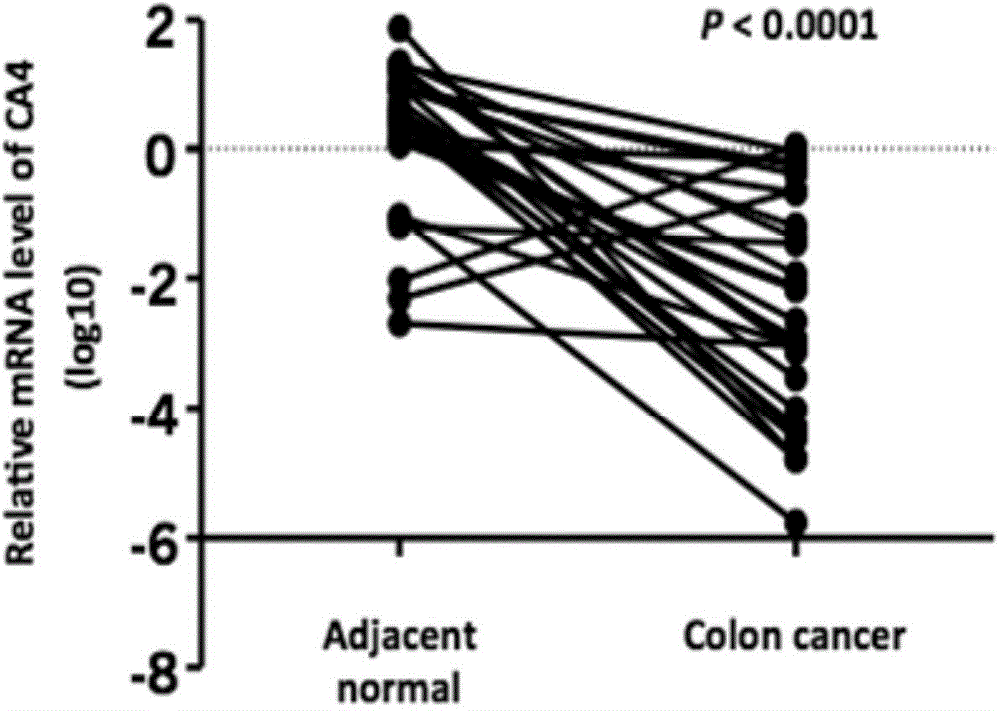
[0044] The mRNA expression level of CA4 in 9 intestinal cancer cell lines: CaCO2, DLD-1, HCT116, HT29, LOVO, LS180, SW480, SW620 and SW1116 was detected by semi-quantitative PCR. The results are as follows: figure 1 as shown, figure 1 To detect the expression level of CA4 mRNA in colon cancer cell lines in Example 1. from figure 1 It can be seen from the results that the mRNA levels of CA4 in these 9 intestinal cancer cells were lower than those in normal int...
Embodiment 2
[0056] In vivo test of CA4 on tumor growth in nude mice:
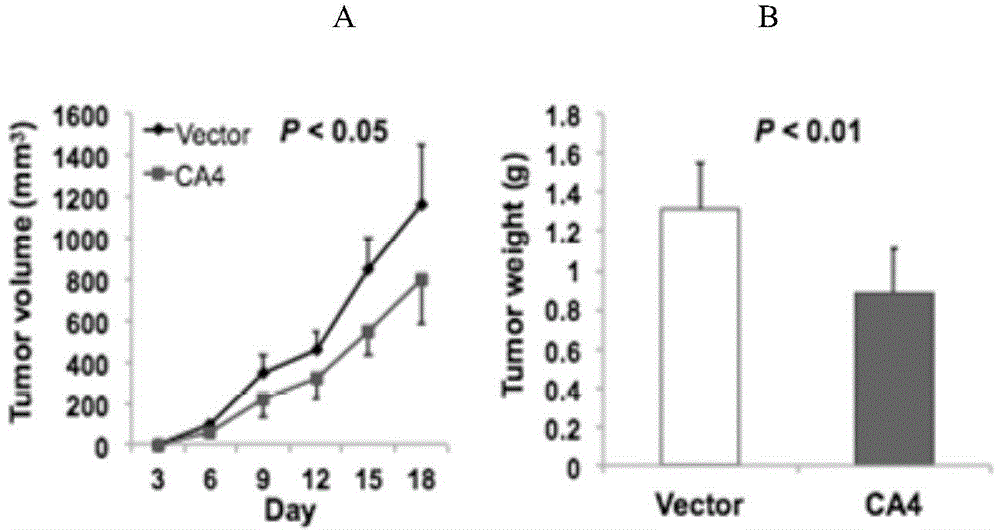
[0057] The control cell lines HCT116 / vector or HCT116 / CA4 were randomly injected into the dorsal side of nude mice, and the tumor growth patterns were compared. Tumor volumes were measured every 3 days for approximately three weeks. Tumor volume (mm3) was calculated by measuring the longest and shortest tumor diameter lengths (volume formula = 0.5 x length x 2 x width). Eighteen days after inoculation, the experiment was terminated and the mice were sacrificed. Tumor growth curves in nude mice Figure 4 shown. The average tumor size of nude mice injected with HCT116 / CA4 was significantly lower than that of control nude mice injected with HCT116 / vector (P image 3 in Part B). like image 3 In part B, the histograms represent the tumor weights of the HCT116 / CA4 and HCT116 / vector groups respectively (P value less than 0.01, t test). Immunostaining with specific antibodies confirmed the expression of CA4 protein in tu...
Embodiment 3
[0059] Intestinal cancer cell CA4 expression level up-regulated after 5-aza-2'-deoxycytidine treatment to identify CA4 methylation as a potential colorectal cancer biomarker test: On the basis of Example 1, in order to determine the role of CA4 in intestinal cancer cells Whether the low expression in is caused by the methylation of the promoter region, we treated 9 kinds of intestinal cancer cells with the DNA methyltransferase inhibitor 5-aza-2'-deoxycytidine: CaCO2, DLD-1, HCT116, HT29 , LOVO, LS180, SW480, SW620 and SW1116; and then the expression of CA4mRNA in cells was detected. Specifically, 1×106 cells were seeded in a 100 mm culture dish and cultured for 24 hours. Experiment with the following groups:
[0060] The control group was the cells without drug addition in the same period;
[0061] The experimental group was cells treated with 2μM 5-Aza-dC for 96h,
[0062] The treated experimental group changed the medium every 24h.
[0063] The experimental results show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com