An alogliptin purifying method
A technology of methylation and dioxo generation, applied in the direction of organic chemistry, can solve the problems of increased cost and operation difficulty, long preparation cycle and complicated operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103]
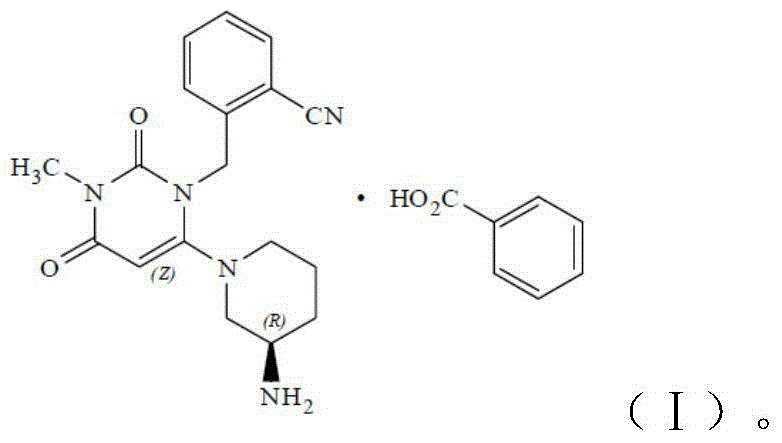
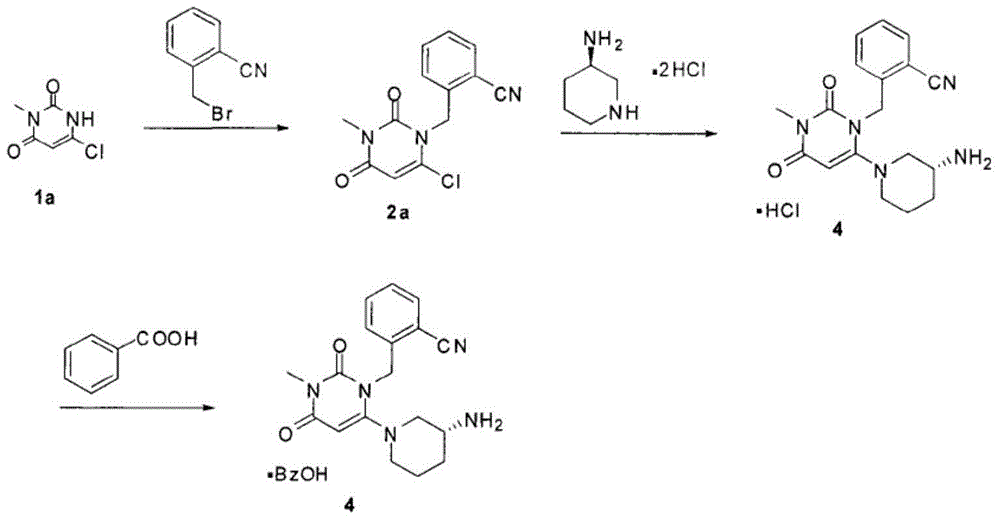
[0104] AG-101 (580g, 2.11mol), (R)-3-aminopiperidine dihydrochloride (400.2g, 2.33mol), sodium bicarbonate (881.6g, 10.50mol) and absolute ethanol (5800ml ) into the three-necked flask of 10000ml, stir well. Raise the temperature to about 70-80°C for reaction, and the reaction is complete in about 5-6 hours (the content of AG-101 is less than 0.1% as monitored by HPLC).
[0105] The above reaction solution was cooled to about 0° C. to about 5° C., stirred for 2 hours, filtered, and the filter cake was washed with absolute ethanol (1160 ml) to obtain filtrate 1.
[0106] The filtrate 1 was concentrated to dryness under reduced pressure in a water bath at about 50°C, and the residue was added with absolute ethanol (580 ml) and stirred at room temperature for crystallization for about 5 hours (add 1 g of seed crystals if there was supersaturation). After a large amount of solids were precipitated, absolute ethanol (5220 ml) was added, and the mixture was stirred at r...
Embodiment 2
[0109] AG-101 (580g, 2.11mol), (R)-3-aminopiperidine dihydrochloride (400.2g, 2.33mol), sodium bicarbonate (881.6g, 10.50mol) and absolute ethanol (5800ml ) into the three-necked flask of 10000ml, stir well. Raise the temperature to about 70-80°C for reaction, and the reaction is complete in about 5-6 hours (the content of AG-101 is less than 0.1% as monitored by HPLC).
[0110] The above reaction solution was cooled to about 0° C. to about 5° C., stirred for about 2 hours, filtered, and the filter cake was washed with absolute ethanol (1160 ml) to obtain filtrate 1.
[0111] The filtrate 1 was concentrated to dryness under reduced pressure in a water bath at about 50°C, and the residue was added with absolute ethanol (5800ml), and stirred at room temperature for about 20 minutes to dissolve the solids, and some suspended solids were insoluble. Activated carbon (58 g) was added and stirred at room temperature for about 10 minutes. Filter under reduced pressure, and wash the ...
Embodiment 3
[0114] AG-101 (580g, 2.11mol), (R)-3-aminopiperidine dihydrochloride (400.2g, 2.33mol), sodium bicarbonate (881.6g, 10.50mol) and absolute ethanol (5800ml ) into the three-necked flask of 10000ml, stir well. Raise the temperature to about 70-80°C for reaction, and the reaction is complete in about 5-6 hours (the content of AG-101 is less than 0.1% as monitored by HPLC).
[0115] The above reaction solution was cooled to room temperature, 58 g of activated carbon was added thereto, stirred for 2 hours, filtered, and the filter cake was washed with absolute ethanol (1160 ml) to obtain filtrate 1.
[0116] The filtrate 1 was concentrated in a water bath at about 50°C to about 20% of the original volume under reduced pressure. A large amount of solids were precipitated, and absolute ethanol (4640ml) was added to dissolve them. Some suspended solids were insoluble, and then activated carbon (58g) was added at room temperature. Stir for about 10 minutes. Filter under reduced press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com