Method for removing thyroxine and triiodothyronine from human serum
A technology for triiodothyronine and thyroxine, applied in the field of serum, can solve the problems of complicated process, excessive material loss and production cost, and achieve the effects of simple operation, low production cost and high clearance rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
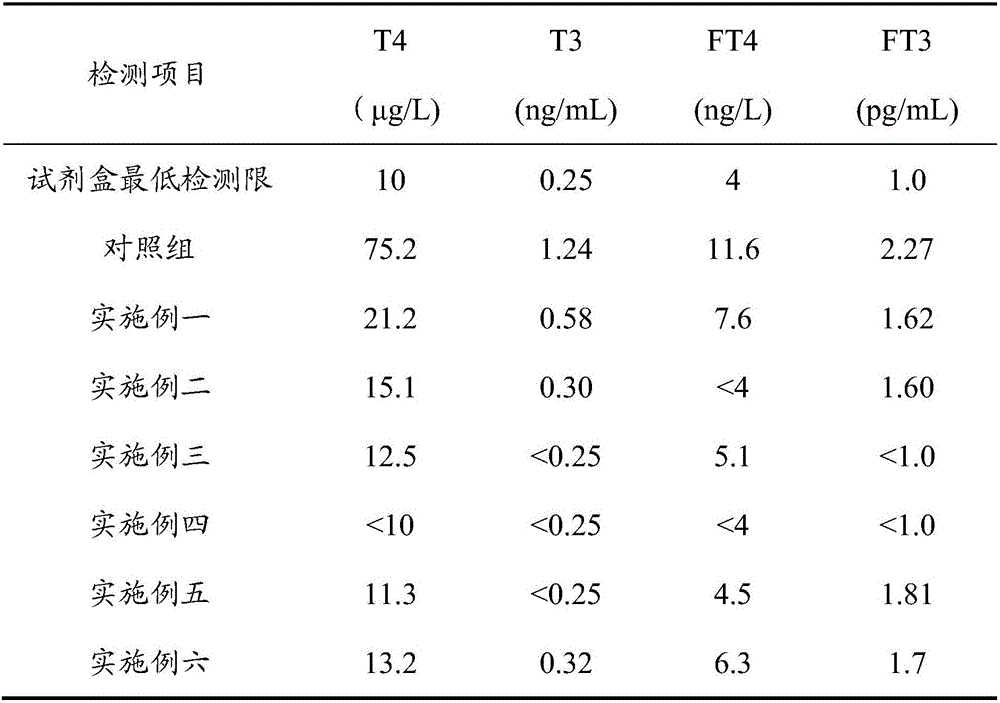
Embodiment 1
[0022] A method for removing thyroxine and triiodothyronine in human serum, comprising the steps of:
[0023] (1) Take human serum, centrifuge at 12000g for 0.2h, and take the supernatant;
[0024] (2) Add 0.05% sodium azide of human serum weight and activated carbon of 8% human serum weight, stir gently at room temperature for 5 hours, centrifuge at 12000g for 0.5 hours, and remove the precipitate;
[0025] (3) Add kaolin with 10% weight of human serum, stir for 15 hours and then centrifuge at 12000g for 0.5 hours to obtain supernatant.
Embodiment 2
[0027] A method for removing thyroxine and triiodothyronine in human serum, comprising the steps of:
[0028] (1) Take the isolated human serum, centrifuge at 12000g for 0.2h, and take the supernatant;
[0029] (2) Add 0.05% human serum weight sodium azide and 8% human serum weight activated carbon with a particle size of 220 mesh, stir gently at room temperature for 5 hours, then centrifuge at 12000g for 0.5 hour to remove the precipitate;
[0030] (3) Add 10% human serum weight kaolin with a particle size of 200 mesh, stir for 15 hours and centrifuge at 12000g for 0.5 hours to obtain supernatant.
[0031] (4) Filter the supernatant obtained in step (3) through 0.8 μm, 0.45 μm and 0.22 μm filter membranes in sequence, and harvest the filtrate.
Embodiment 3
[0033] A method for removing thyroxine and triiodothyronine in human serum, characterized in that it comprises the steps:
[0034] (1) Take human serum, centrifuge at 16000g for 1.0h, and take the supernatant;
[0035] (2) Add 0.08% human serum weight sodium azide and 15% human serum weight activated carbon with a particle size of 180 mesh, stir gently at room temperature for 16 hours, then centrifuge at 16000g for 2 hours to remove the precipitate;
[0036] (3) Add 20% human serum weight kaolin with a particle size of 150 mesh, stir for 30 hours and then centrifuge at 16000 g for 2 hours to obtain the supernatant.
[0037] (4) Filter the supernatant obtained in step (3) through 0.8 μm, 0.45 μm and 0.22 μm filter membranes in sequence, and harvest the filtrate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com