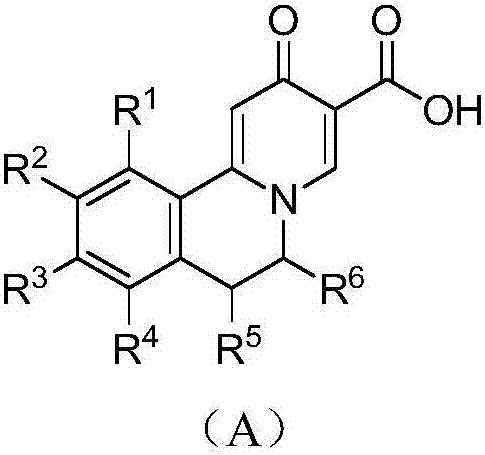
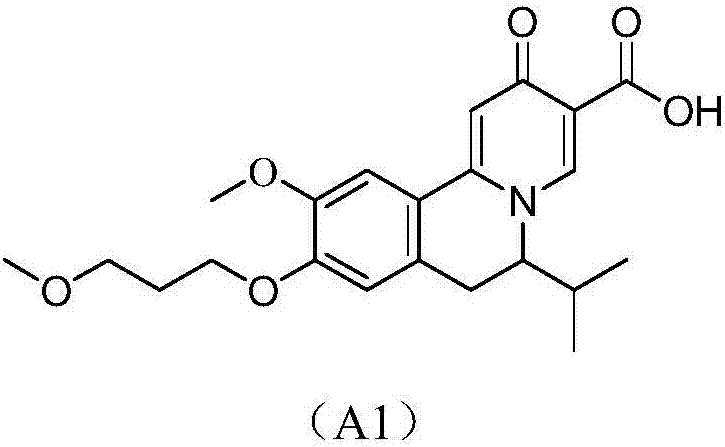
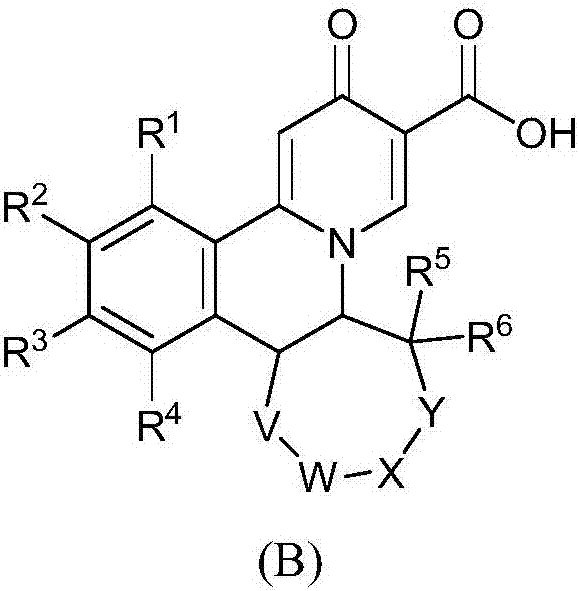
Quinolizinones compound and preparation method and application thereof
The technology of a compound, quinazinone, applied in the field of medicine, can solve the problem of unknown anti-HBV activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1c
[0065] Example 1cis-10-methoxy-11-(3-methoxypropoxy)-3,3-dimethyl-7-oxo-3,3a,7,12b-tetrahydro-2H-furan And[3,2-c]pyrido[2,1-a]isoquinazine-6-carboxylic acid
[0066] The synthetic route is as follows:
[0067]
[0068] Step 1: Preparation of 6-(2-Hydroxy-1,1-dimethyl-ethyl)-10-methoxy-9-(3-methoxypropoxy)-2-oxo-6,7 -Ethyl dihydrobenzo[a]quinazine-3-carboxylate
[0069] 6-(2-Benzyloxy-1,1-dimethyl-ethyl)-10-methoxy-9-(3-methoxypropoxy)-2-oxo-6,7- Ethyl dihydrobenzo[a]quinazine-3-carboxylate (1.65g, 3mmol, preparation reference patent document US20160122344A1), and Pd / C (10%) (0.5g) were slowly added in ethanol (200mL), in hydrogen Pressurized under the atmosphere and mechanically stirred to obtain crude 6-(2-hydroxy-1,1-dimethyl-ethyl)-10-methoxy-9-(3-methoxypropoxy)-2 -Ethyl oxo-6,7-dihydrobenzo[a]quinazine-3-carboxylate (1.10 g, yield about 79.7%) was directly used in the next step without purification.
[0070] 1 H NMR (400MHz, CDCl 3 )δ:8.43(s,1H),7.12(s,1H),6.89(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com