An RT-HPLC detecting method for valdecoxib/parecoxib related substances
An RT-HPLC, detection method technology, applied in the field of drug analysis, can solve the problem of inability to separate valdecoxib valdecoxib characteristic peaks, inability to distinguish between parecoxib characteristic peaks and parecoxib isomers. Separation of characteristic peaks of meta-parecoxib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Instrument and chromatographic conditions
[0037] High performance liquid chromatography: Agilent1260 high performance liquid chromatography system and workstation;
[0038] Chromatographic column: phenyl column (SUPELCOSILLC-DP250*4.6mm, 5μm);
[0039] Prepare a 0.01mol / L disodium hydrogen phosphate solution, adjust the pH value to 3.0 with phosphoric acid as the water phase, the ratio of water phase to methanol in the mobile phase is 52:48, set the flow rate to 1.0ml / min, and the detection wavelength to 215nm. The temperature is 40°C.
[0040] (2) Experimental steps
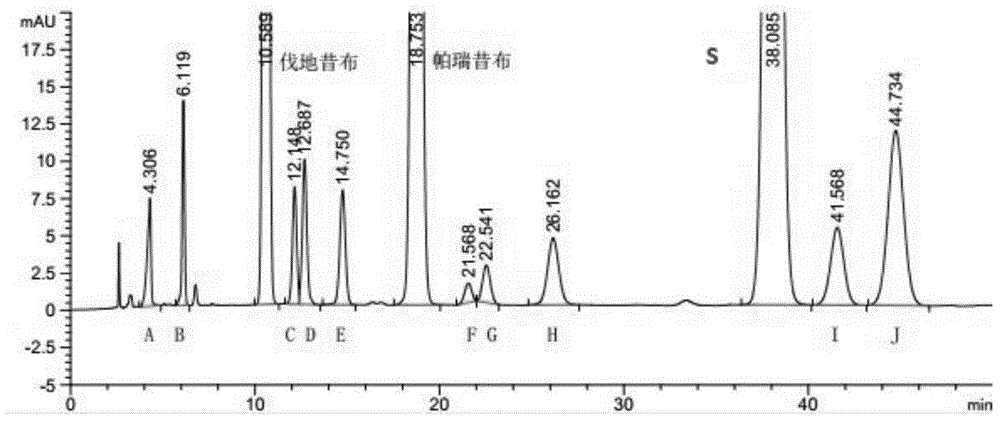
[0041] Take parecoxib sodium, meta-parecoxib (G), parecoxib isomers (F), valdecoxib, meta-valdecoxib (D), valdecoxib Isomer (C), hydrolysis impurity (A), disubstituted impurity (B), dimer impurity (H), acetylation impurity (E), valdecoxibate ethyl ester (J), starting Appropriate amount of material S, S isomer (I), dissolved and diluted with methanol-water (1:1) to make about 0.2 mg each of valde...
Embodiment 2
[0044] (1) Instrument and chromatographic conditions
[0045] High performance liquid chromatography: Agilent1260 high performance liquid chromatography system and workstation;
[0046] Chromatographic column: phenyl column (SUPELCOSILLC-DP250*4.6mm, 5μm);
[0047] Prepare 0.01mol / L disodium hydrogen phosphate solution, adjust the pH value to 3.0 with phosphoric acid as the water phase, the ratio of water phase to methanol in the mobile phase is 55:45, set the flow rate to 1.0ml / min, and the detection wavelength to 215nm. The temperature is 40°C.
[0048] (2) Experimental steps
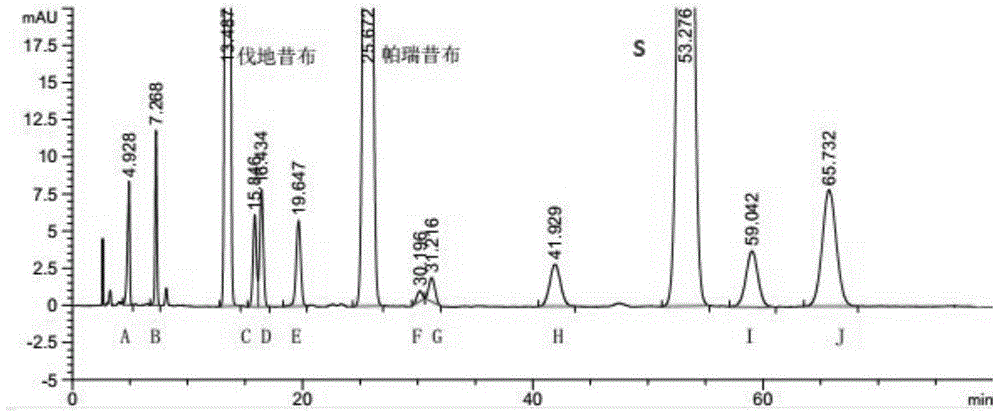
[0049] Take parecoxib sodium, meta-parecoxib (G), parecoxib isomers (F), valdecoxib, meta-valdecoxib (D), valdecoxib Isomer (C), hydrolysis impurity (A), disubstituted impurity (B), dimer impurity (H), acetylation impurity (E), valdecoxibate ethyl ester (J), starting Appropriate amount of material S, S isomer (I), dissolved and diluted with methanol-water (1:1) to make about 0.2 mg each of valdeco...
Embodiment 3
[0052] (1) Instrument and chromatographic conditions
[0053] High performance liquid chromatography: Agilent1260 high performance liquid chromatography system and workstation;
[0054] Chromatographic column: phenyl column (SUPELCOSILLC-DP250*4.6mm, 5μm);
[0055] Prepare a 0.01mol / L disodium hydrogen phosphate solution, adjust the pH value to 3.0 with phosphoric acid as the water phase, and carry out gradient elution with the water phase-methanol ratio in the mobile phase as shown in Table 2, set the flow rate to 1.0ml / min, and detect the wavelength at 215 nm, the column temperature is 40°C.
[0056] Table 2
[0057]
water box%
The organic phase%
0min
52
48
25min
52
48
40min
40
60
40.1min
52
48
50min
52
48
[0058] (2) Experimental steps
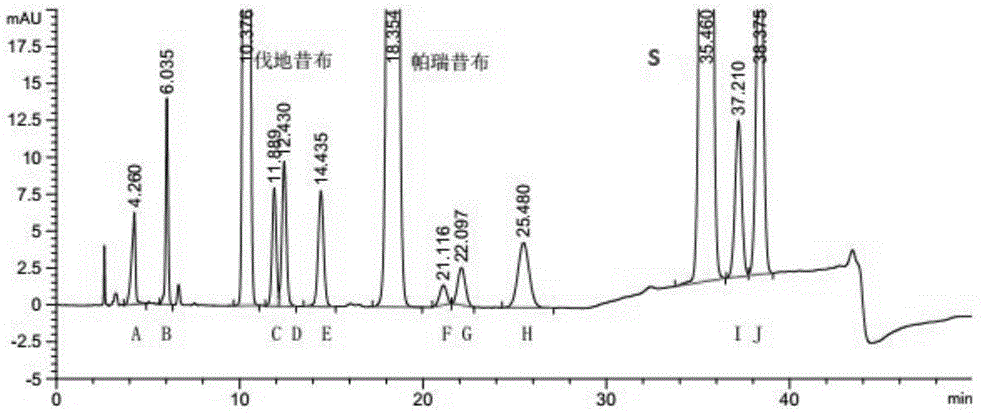
[0059] Take parecoxib sodium, meta-parecoxib (G), parecoxib isomers (F), valdecoxib, meta-valdecoxib (D), valdecoxib Isomer (C), h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com