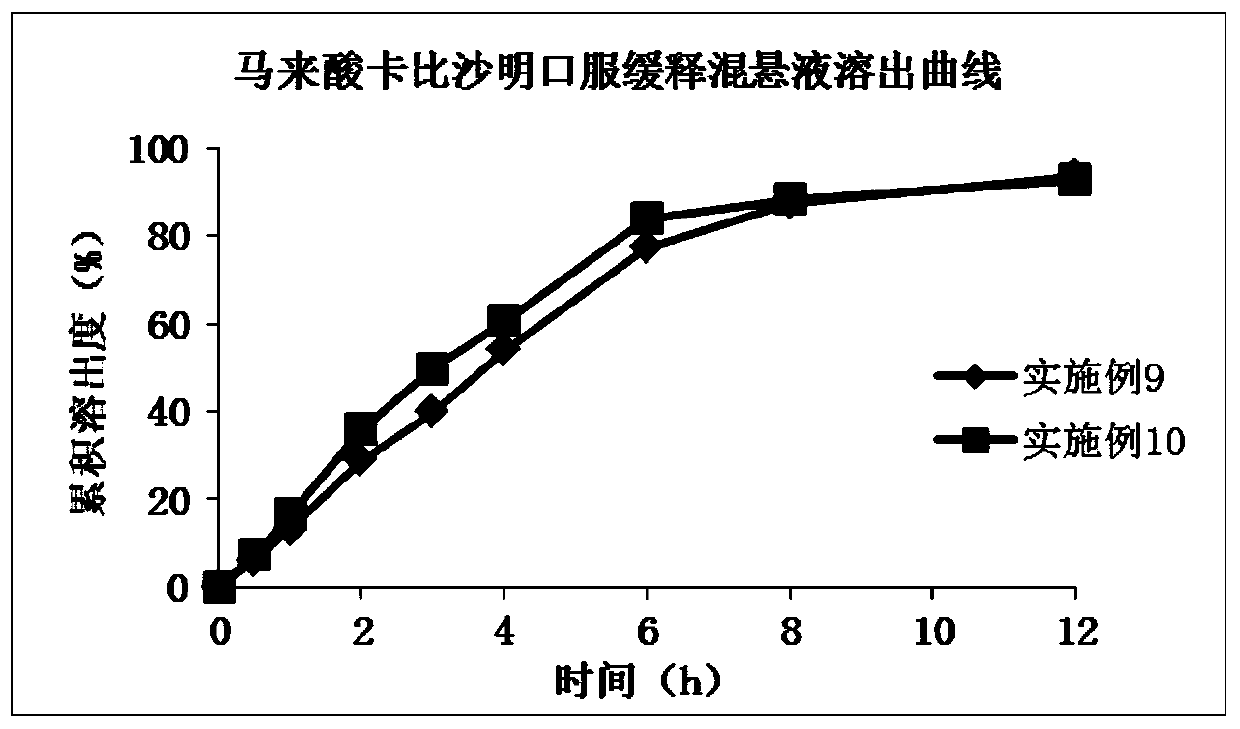
A kind of liquid sustained-release preparation and preparation method thereof
A slow-release preparation and liquid technology, applied in the field of medicine, can solve the problems of low drug yield, poor coating reproducibility, difficult industrialization, etc., and achieve the effects of reducing the peak concentration of the drug, complete coating, and conducive to scale-up production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] [embodiment 1] the preparation of ambroxol hydrochloride-resin complex
[0051] (1) Dissolve 400g of Ambroxol hydrochloride in 4L of deionized water; (2) Slowly add 8000g of Amberlite under continuous mixing TM IRP-69 resin, at 50°C, continuously stirred for 12 hours, let it stand, and poured off the supernatant to obtain the drug-resin complex; (3) Add 4L deionized water to the drug-resin complex obtained in (2) , stirred for 10 min, washed off the unbound free drug, left to stand, and poured off the supernatant; repeat step (3) twice; (4) dry the drug-resin complex obtained in (3) at 60°C, That is, the drug-resin complex is obtained.
Embodiment 2
[0052] [embodiment 2] the preparation of the impregnated acyclovir-resin complex
[0053] (1) Dissolve 140g of acyclovir in 20L of 0.01mol / L hydrochloric acid solution; (2) Slowly add 1400g of JK006 ion exchange resin under continuous mixing, stir continuously for 8h at 25°C, let stand, and pour off the clear liquid to obtain the drug-resin complex; (3) add 20L deionized water to the drug-resin complex obtained in (2), stir for 30min, wash off the unbound free drug, let it stand, and pour off the supernatant ; Repeat step (3) to operate 4 times; (4) dry the drug-resin complex obtained in (3) to a water content of 20% at 60°C; (5) add 2.6g triacetin to 165g Kollicoat SR 30D In the polymer, stir at room temperature for 1 h to obtain an impregnating agent; (6) place the drug-resin compound obtained in step (4) in a wet granulator, add 167.6 g of impregnating agent to the drug-resin compound, stir, and shear , to form a uniform material; (7) drying the impregnated drug-resin comp...
Embodiment 3
[0054] [embodiment 3] the preparation of the impregnated topiramate-resin complex
[0055] (1) Dissolve 200g of Topiramate in 40L of deionized water; (2) Slowly add 10g of Amberlite with continuous mixing TM IRP-69 ion exchange resin, at 40°C, continuously stirred for 2 hours, let it stand, and poured off the supernatant to obtain the drug-resin complex; (3) Add 40 L to the drug-resin complex obtained in (2). Ionized water, stirring for 30min, washing away unbound free drug, standing still, decanting the supernatant; repeating step (3) for 3 times; (4) placing the drug-resin complex obtained in (3) at 40°C Dry to a water content of 3%; (5) add 5.3g triacetin to 334g Kollicoat SR 30D polymer, stir at room temperature for 1h to obtain an impregnating agent; (6) place the drug-resin complex obtained in step (4) in a wet In the French granulator, add 340g of impregnating agent into the drug-resin compound, stir and shear to form a uniform material; (7) dry the impregnated drug-re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com