A anti -allergic drug mixed -release suspension and preparation method
A slow-release suspension and anti-allergic technology, which is applied in the direction of drug combination, pharmaceutical formula, allergic diseases, etc. It can solve the problems of uncontrollable drug release, high energy consumption of drug dissolution, and increased energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
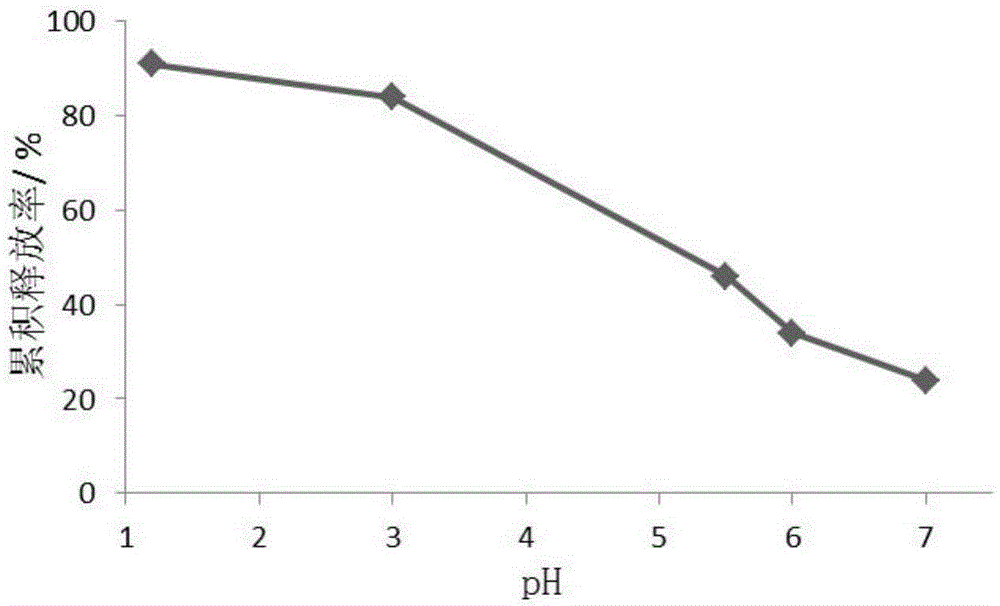
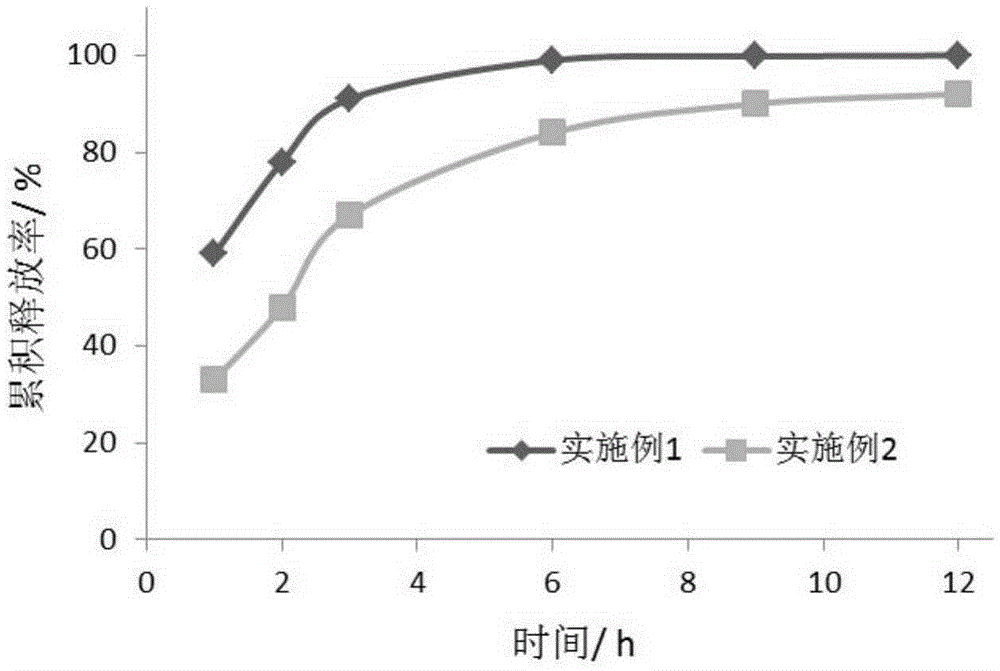
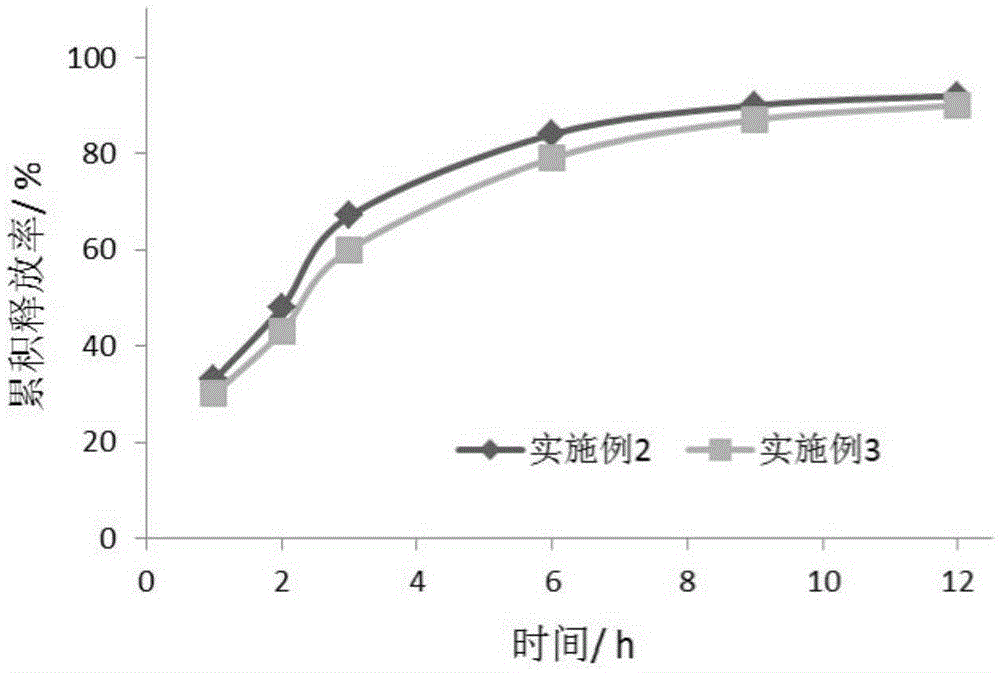
Image
Examples
Embodiment 1
[0069] Example 1 Preparation of drug-ion exchange resin complex.
[0070] Preparation steps: (1): Dissolve 166.67g of carbinoxamine maleate in 1L of purified water; (2): Take 500g of Amberlire IRP-69 resin passed through a 80-mesh sieve, and add it to the solution obtained in (1) while stirring , stirred continuously for 4 hours, stood still, and filtered to obtain a jelly; (3): Add 2L of purified water to the jelly obtained in step (2), stir slowly for 10min, remove the supernatant, and repeat step (3) once ; (4): Dry the drug-resin complex obtained in (3) at 45°C to a water content of 5%, and store in a sealed and dry place.
Embodiment 2
[0071] Example 2 Preparation of drug-containing core.
[0072] Preparation steps: (1): Dissolve 166.67g of carbinoxamine maleate in 1L of purified water; (2): Take 500g of Amberlire IRP-69 resin passed through a 80-mesh sieve, and then add it to the solution obtained in (1) while stirring , continuously stirred for 4 hours, stood still, and filtered to obtain a jelly; (3): Add 2L of purified water to the jelly obtained in (2), stir slowly for 10min, remove the supernatant, and repeat step (3) once ; (4): The drug-resin complex obtained in (3) was dried at 45°C until the water content was 50%; (5): 15.00 g of sodium alginate passed through an 80-mesh sieve and 3.22 g of sodium alginate passed through an 80-mesh sieve Slowly add povidone into the drug-resin complex obtained in (4) under stirring, and stir evenly; (6): Dry the mixture obtained in (5) at 45°C to a water content of 25%, and grind it with a grinder for about 10 minutes , passed through a 80-mesh sieve, then dried a...
Embodiment 3
[0073] Example 3 Preparation of drug-containing core.
[0074] Preparation steps: (1): Dissolve 166.67g of carbinoxamine maleate in 1L of purified water; (2): Mix 500.00g of AmberlireIRP-69 and 15.00g of sodium alginate resin passed through an 80-mesh sieve, and then stir Add to the solution obtained in (1), stir continuously for 4 hours, let it stand, and filter to obtain a jelly; (3): Add 2L of purified water to the jelly obtained in (2), stir slowly for 10min, remove the supernatant, repeat Step (3) is operated once; (4): Dry the jelly obtained in (3) at 45°C until the water content is 50%; (5): Mix 3.22g of methyl cellulose passed through a 80-mesh sieve Slowly add it to the drug jelly obtained in (4), and stir evenly; (6): Dry the mixture obtained in (5) at 45°C until the water content is 25%, grind it with a grinder for about 10 minutes, and pass it through a 80-mesh sieve. Then dry at 45°C to a water content of 5%, grind for 10 minutes with a grinder, and pass through an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com