Application of compounds Pyrrocidines in preparation of anti-tuberculosis drug
A compound and tuberculosis technology, applied in the field of biology, can solve the problem of lack of new anti-tuberculosis drug screening and other problems, and achieve the effect of high drug availability, abundant sources and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Fusion expression of Mycobacterium tuberculosis tyrosine phosphatase B
[0026] (1) Gene cloning of Mycobacterium tuberculosis tyrosine phosphatase B, proceed as follows:
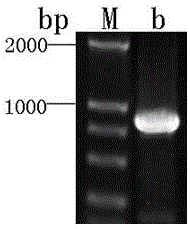
[0027] According to the DNA sequence that inhibits Mycobacterium tuberculosis tyrosine phosphatase B, design and synthesize a pair of primers, add NdeⅠ, XhoⅠ restriction sites to the upstream and downstream primers, and use the genomic DNA of Mycobacterium tuberculosis H37Ra as Template, the 831bp long DNA fragment of Mycobacterium tuberculosis tyrosine phosphatase B was amplified by PCR with the above primers. Electrophoresis and recovers PCR amplified DNA fragments.
[0028] (2) Construction of a vector expressing Mycobacterium tuberculosis tyrosine phosphatase B:
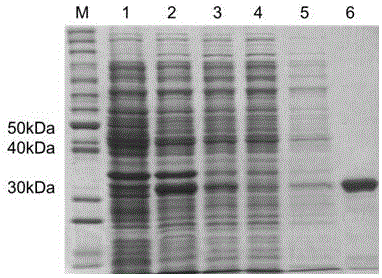
[0029] The plasmid pET28a(+) was extracted, and the DNA fragments of the plasmid and Mycobacterium tuberculosis tyrosine phosphatase B were digested with NdeI and XhoI, and recovered after electrophoresis. The recovered product was ...
Embodiment 2
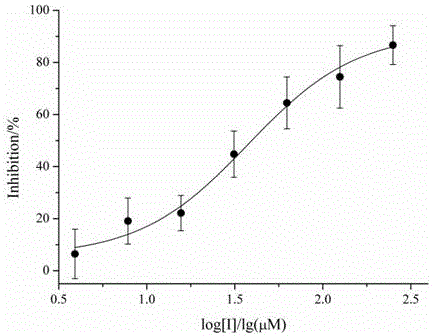
[0036] Example 2 Analysis of inhibitory enzyme activity of compound Pyrrocidines on Mycobacterium tuberculosis tyrosine phosphatase B
[0037] The enzyme activity of recombinant Mycobacterium tuberculosis tyrosine phosphatase B was analyzed using disodium p-nitrophenyl phosphate (pNPP) as the substrate. The Mycobacterium tuberculosis tyrosine phosphatase B used was purified in Example 1. The recombinant Mycobacterium tuberculosis tyrosine phosphatase B fusion protein. The 500mMpNPP stock solution stored at -20°C is dissolved on ice, and pNPP and MptpB are diluted appropriately with reaction buffer (50mMTris, 100mMNaCl, pH7.8). In a 96-well plate, add MptpB at a final concentration of 6μg / mL, a concentration gradient of compounds (0μM, 3.9μM, 7.8μM, 15.6μM, 31.25μM, 62.5μM, 125μM, 250μM), 1.5mM pNPP, total reaction The volume is 200 μL. React at 37°C for 5 min, and read the absorbance value of each well at 405 nm by a light absorption microplate reader. According to the absorba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com