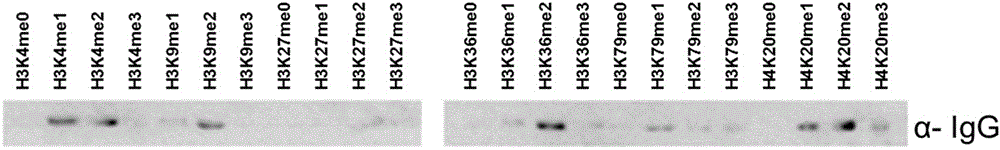
Generic methylation and dimethylation antibody and preparation method thereof
A dimethylation and methylation technology, applied in the field of biomedicine, can solve problems such as the lack of antibodies that specifically recognize trimethylated proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
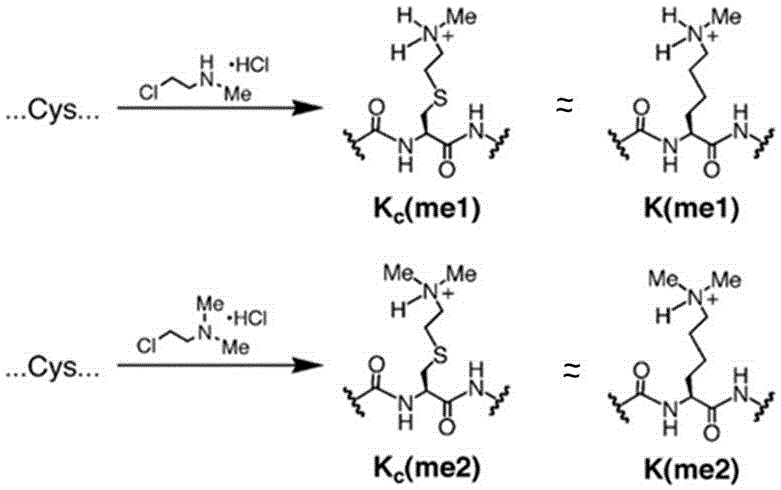
[0028] One: Preparation of bovine serum albumin BSA that simulates cysteine into monomethylated lysine (Kc[me1])
[0029] 1: Dissolve 10mg BSA bovine serum albumin BSA in 900ul alkylation reagent (1M 4-hydroxyethylpiperazineethanesulfonic acid, pH7.8; 4M guanidine hydrochloride 10mML-methionine);
[0030] 2: Add 20ul of 1M dithiothreitol (DTT), and react at 37°C for 1 hour;
[0031] 3: Add 100ul of 1M (2-chloroethyl)-methylammonium chloride, and carry out alkylation reaction at room temperature for 4 hours;
[0032] 4: Add 10ul1M dithiothreitol (DTT) and react at room temperature for 10 hours;
[0033] 5: Add 50ul of 14.2M mercaptoethanol (BME) to terminate the reaction to obtain simulated monomethylated bovine serum albumin BSA (Kc[me1]).
[0034] Two: Preparation of bovine serum albumin BSA that simulates cysteine into dimethylated lysine (Kc[me2])
[0035] 1: Dissolve 10mg BSA bovine serum albumin BSA into 930ul alkylation reagent (1M 4-hydroxyethylpiperazineethanesu...
Embodiment 2
[0044] 1: Preparation of hemocyanin KLH that simulates cysteine into monomethylated lysine (Kc[me1])
[0045] 1: Weigh 10mg hemocyanin KLH and dissolve it into 900ul alkylation reagent (1M 4-hydroxyethylpiperazineethanesulfonic acid, pH7.8; 4M guanidine hydrochloride 10mML-methionine);
[0046] 2: Add 20ul of 1M dithiothreitol (DTT), and react at 37°C for 1 hour;
[0047] 3: Add 100ul of 1M (2-chloroethyl)-methylammonium chloride, and carry out alkylation reaction at room temperature for 4 hours;
[0048] 4: Add 10ul1M dithiothreitol (DTT) and react at room temperature for 10 hours;
[0049]5: Add 50 ul of 14.2 M mercaptoethanol (BME) to terminate the reaction to obtain hemocyanin KLH (Kc[me1]) which simulates monomethylation modification.
[0050] Two: Preparation of hemocyanin KLH that simulates cysteine into dimethylated lysine (Kc[me2])
[0051] 1: Weigh 10mg hemocyanin KLH and dissolve it in 930ul alkylation reagent (1M 4-hydroxyethylpiperazineethanesulfonic acid, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com