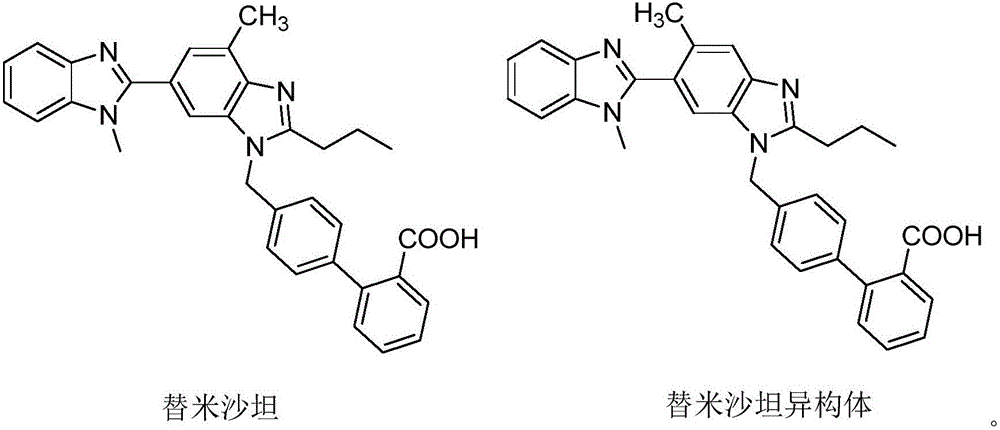
Telmisartan tablet
A technology of telmisartan and calcium dihydrogen phosphate, which is applied in the field of pharmaceutical preparations, can solve the problems that the two cannot be separated, and achieve the effects of high quality stability, strong moisture resistance, and good dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
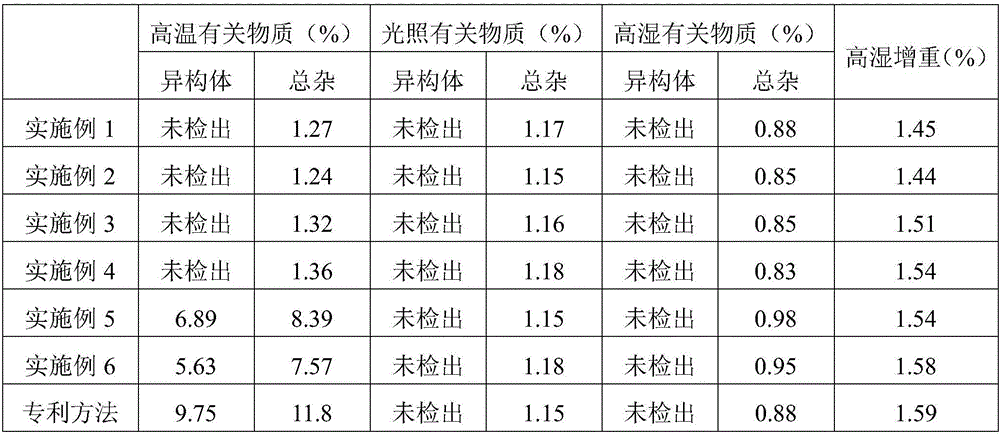
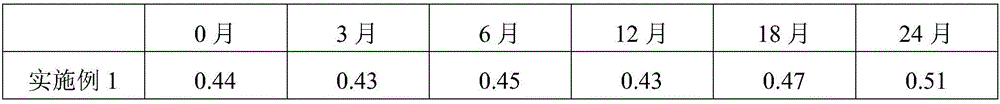
Embodiment 1
[0027] Embodiment 1: the preparation of telmisartan tablet
[0028] Group ratio:
[0029] 100 parts of telmisartan, 8.5 parts of potassium hydroxide, 30 parts of meglumine, 340 parts of sorbitol, 85 parts of a mixture of calcium hydrogen phosphate and calcium dihydrogen phosphate, 25 parts of povidone, and 12 parts of magnesium stearate; Wherein, the weight ratio of calcium hydrogen phosphate and calcium dihydrogen phosphate is 9.5:1.
[0030] Preparation:
[0031] Step S1: first take potassium hydroxide and meglumine, add water to dissolve; then add telmisartan, add ethanol, shake to dissolve telmisartan, and dry under reduced pressure at 40°C to obtain a white bulky solid, which is crushed through 200 mesh Sieve to get telmisartan salt powder;
[0032] Step S2: Sorbitol, calcium hydrogen phosphate, calcium dihydrogen phosphate and povidone are pulverized through a 100-mesh sieve, magnesium stearate is passed through a 40-mesh sieve, and mixed with telmisartan salt powd...
Embodiment 2
[0034] Embodiment 2: the preparation of telmisartan tablet
[0035] Group ratio:
[0036] 100 parts of telmisartan, 8.5 parts of potassium hydroxide, 30 parts of meglumine, 340 parts of sorbitol, 85 parts of a mixture of calcium hydrogen phosphate and calcium dihydrogen phosphate, 25 parts of povidone, and 12 parts of magnesium stearate; Wherein, the weight ratio of calcium hydrogen phosphate and calcium dihydrogen phosphate is 9.5:1.
[0037] Preparation:
[0038] Step S1: first take potassium hydroxide and meglumine, add water to dissolve; then add telmisartan, add ethanol, shake to dissolve telmisartan, and spray dry to obtain telmisartan salt powder;
[0039] Step S2: Sorbitol, calcium hydrogen phosphate, calcium dihydrogen phosphate and povidone are pulverized through a 100-mesh sieve, magnesium stearate is passed through a 40-mesh sieve, and mixed with telmisartan salt powder evenly;
[0040] Step S3: compress the mixed powder into tablets to obtain Telmisartan tab...
Embodiment 3
[0041] Embodiment 3: the preparation of telmisartan tablet
[0042] Group ratio:
[0043] 90 parts of telmisartan, 8 parts of potassium hydroxide, 25 parts of meglumine, 320 parts of sorbitol, 80 parts of a mixture of calcium hydrogen phosphate and calcium dihydrogen phosphate, 20 parts of povidone, and 10 parts of magnesium stearate; Wherein, the weight ratio of calcium hydrogen phosphate and calcium dihydrogen phosphate is 9:1.
[0044] Preparation:
[0045] Step S1: first take potassium hydroxide and meglumine, add water to dissolve; then add telmisartan, add ethanol, shake to dissolve telmisartan, and dry under reduced pressure at 40°C to obtain a white bulky solid, which is crushed through 200 mesh Sieve to get telmisartan salt powder;
[0046] Step S2: Sorbitol, calcium hydrogen phosphate, calcium dihydrogen phosphate and povidone are pulverized through a 100-mesh sieve, magnesium stearate is passed through a 40-mesh sieve, and mixed with telmisartan salt powder ev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com