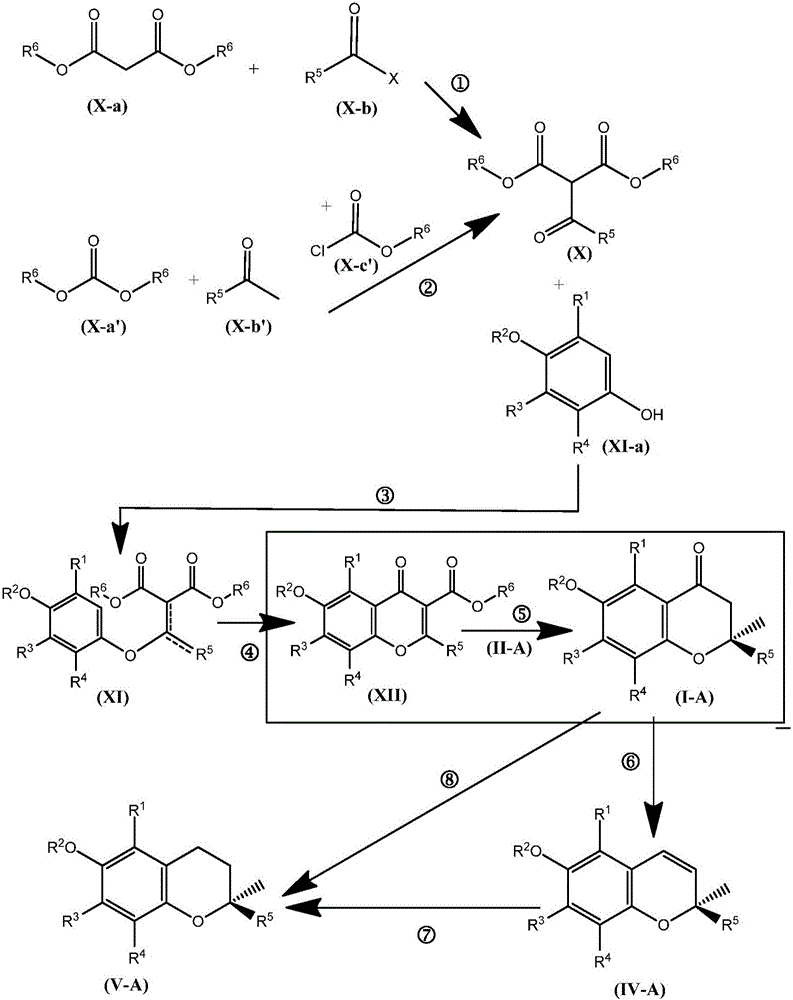
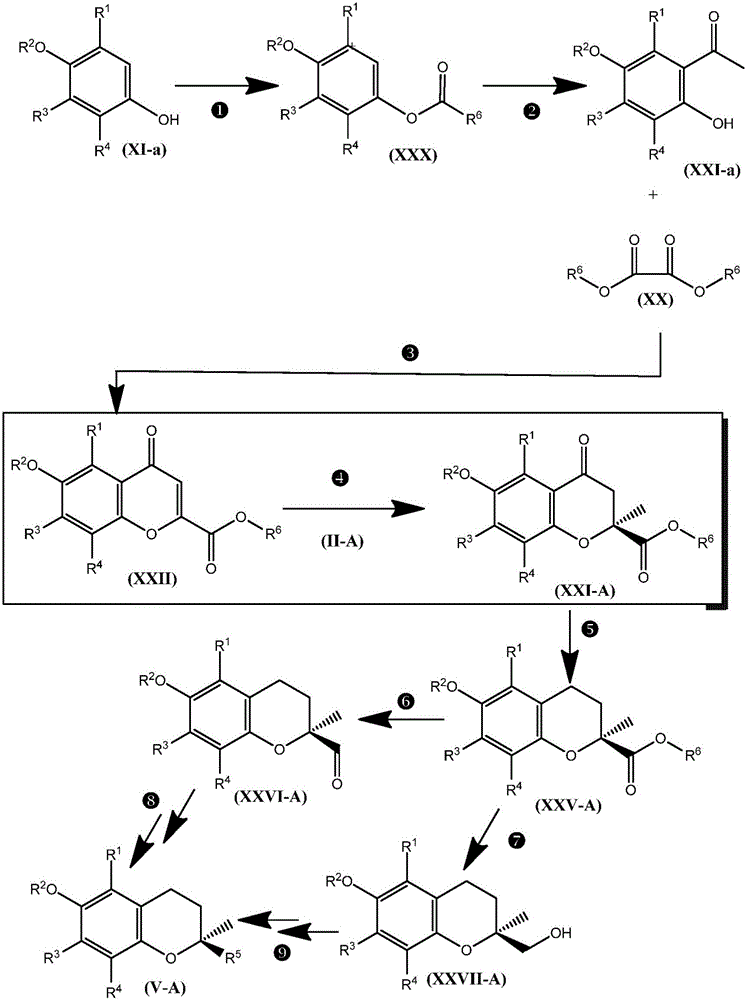
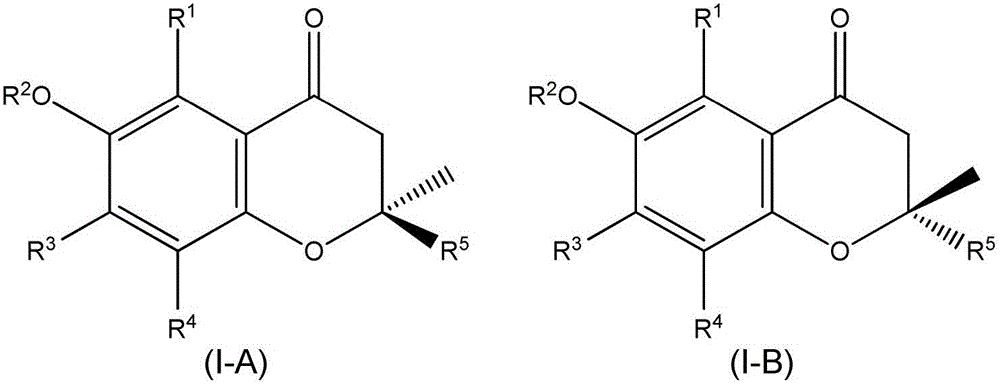
Synthesis of chromane compounds and their derivatives by a copper-catalyzed conjugate addition reaction
A compound and chiral compound technology, applied in the field of synthesis of chiral chroman compounds and their derivatives and precursors, can solve problems such as difficult stereoselective realization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0245] The present invention is further illustrated by the following experiments.
[0246] Preparation of (7R,11R)-7,11,15-trimethyl-3-oxocetanoic acid methyl ester (②)
[0247]
[0248] NaH (8.94 g, 223.5 mmol, 60% in paraffin oil, 3.0 eq.) and KH (1.08 g, 13.72 mmol, 50% in paraffin oil, 0.18 eq.) were washed with n-hexane (3x) and removed in vacuo. Remove residual solvent. The solid residue was suspended in THF (90 mL), then dimethyl carbonate (18.8 mL, 223.5 mmol, 3.0 eq.) was added. The resulting suspension was heated to reflux, then 1 mL of (6R, 1OR)-trimethylpentadecan-2-one (24.1 mL, 20.0 g, 74.5 mmol, 1.0 eq.) was added via the dropping funnel (according to WO2012 / 171969A1 in Example 19 synthesis) in THF (tetrahydrofuran) (60 mL). After 15 min, the remaining solution of (6R,10R)-trimethylpentadecan-2-one was added dropwise. Subsequently, the reaction was heated to reflux for 4 hours. After cooling by an ice bath, the reaction was quenched by the addition of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com