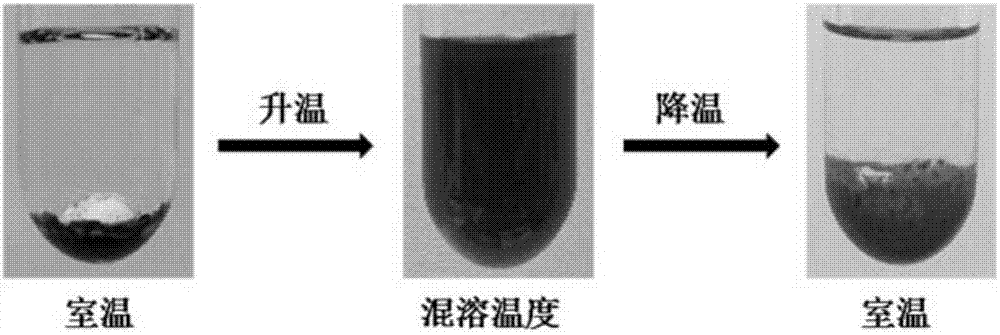
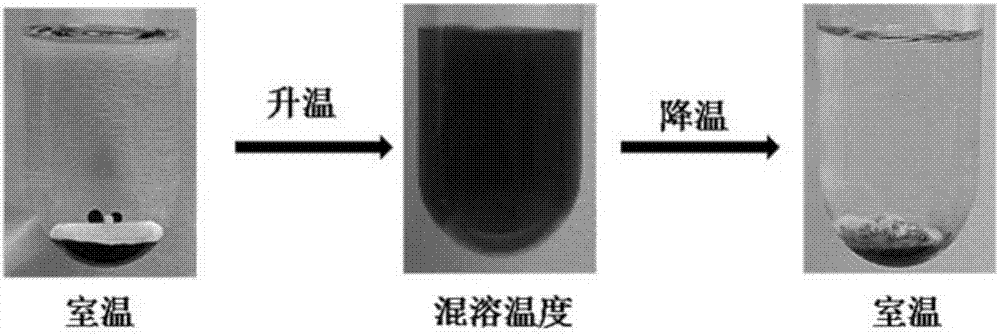
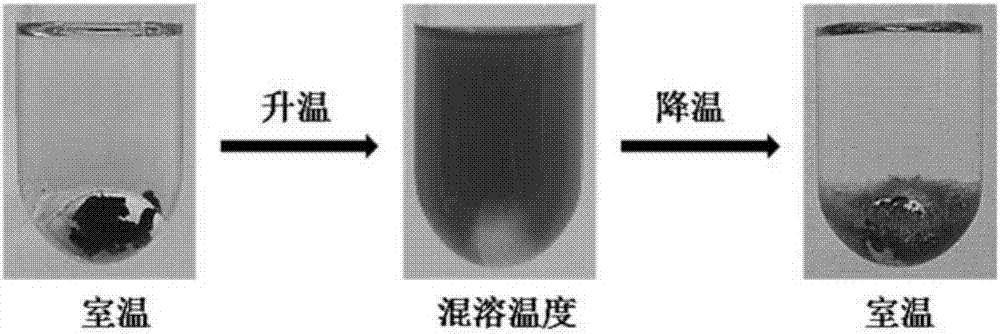
chiral ionic liquid
A chiral ionic liquid and phase separation technology, applied in asymmetric synthesis, organic compound/hydride/coordination complex catalysts, preparation of organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The present invention further discloses a preparation method of the above-mentioned chiral ionic liquid, comprising the following steps:
[0023] (1) The chemical formula is CH 3 (OCH 2 CH 2 ) n The chemical formula is CH 3 (OCH 2 CH 2 ) n OSO 2 CH 3 Polyethylene glycol monomethyl ether sulfonate; This step is implemented in the following manner: Polyethylene glycol monomethyl ether CH 3 (OCH 2 CH 2 ) n OH and triethylamine are dissolved in toluene at a molar ratio of 1:1 to 1.2, and methanesulfonyl chloride 1 to 1.5 times the molar number of polyethylene glycol monomethyl ether is added dropwise in an ice-water bath, and stirred after the dropwise addition Overnight; the white solid was removed by filtration, and the resulting filtrate was treated to obtain polyethylene glycol monomethyl ether sulfonate CH 3 (OCH 2 CH 2 ) n OSO 2 CH 3 ;
[0024] (2) The obtained polyethylene glycol monomethyl ether sulfonate CH 3 (OCH 2 CH 2 ) n OSO 2 CH 3 The ...
Embodiment 1
[0036] The synthetic method of chiral ionic liquid i (n=16), comprises the steps:
[0037]
[0038] (1) Polyethylene glycol monomethyl ether CH 3 (OCH 2 CH 2 ) n OH (n=16) and triethylamine were dissolved in toluene at a molar ratio of 1:1, and methanesulfonyl chloride, which was equivalent to 1 molar equivalent of polyethylene glycol monomethyl ether, was added dropwise in an ice-water bath until the addition was complete. Stir overnight. The white solid was removed by suction filtration under reduced pressure, and the obtained colorless transparent filtrate was processed to obtain the corresponding polyethylene glycol monomethyl ether sulfonate CH 3 (OCH 2 CH 2 ) 16 OSO 2 CH 3 . 1 H NMR (400MHz, CDCl 3 ):δ3.1(s,3H,OSO 2 CH 3 ),δ3.38(s,3H,OCH 3 ), δ3.56~3.88(t,64H,(OCH 2 CH 2 ).
[0039] (2) the above obtained polyethylene glycol monomethyl ether sulfonate CH 3 (OCH 2 CH 2 ) 16 OSO 2 CH 3 With cinchonidine in a molar ratio of 1:1.2, reflux and stir i...
Embodiment 2
[0041] The synthetic method of chiral ionic liquid ii (n=12)
[0042]
[0043] The raw material is polyethylene glycol monomethyl ether CH 3 (OCH 2 CH 2 ) n OH (n=12), others The chiral ionic liquid ii was synthesized by the same synthesis method as in Example 1. 1 H NMR (400MHz, CDCl 3 ):δ3.1(s,3H,OSO 2 CH 3 ),δ3.38(s,3H,OCH 3 ), δ3.56~3.88(t,48H,(OCH 2 CH 2 ). 1 H NMR (400MHz, CDCl 3 ):δ8.6(d,1H,NCH),δ8.2(d,1H,CCHCH),δ7.9(d,1H,NCCHCH),δ7.8(t,1H,NCCHCH),δ7.6( t,1H,NCCHCHCH),δ7.4(d,1H,NCHCH),δ5.7(m,1H,CH 2 CH),δ5.0(d,2H,CH 2 CH),δ5.1(d,1H,CHOH),δ4.0(q,1H,N + CHCHOH), δ3.8(q,2H,N + CH 2 CH 2 O), δ3.6(s, 1H, OH), δ3.4~3.7(q, 44H, OCH 2 CH 2 O), δ3.3(s, 3H, CH 3 O), δ3.1~3.4(d,2H,N + CH 2 CHCHCH 2 ;q,2H,N + CH 2 CH 2 ;q,2H,N + CH 2 CH 2 O),δ2.84(s,3H,CH 3 SO 3 - ),δ2.78(m,1H,CH 2 CHCH), δ1.5~1.8(m,5H,N + CH 2 CH 2 CH,N + CH 2 CH 2 ,N + CHCH 2 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com