Use of cinchonine in preparation of anti-tumor drugs
An anti-tumor drug, the technology of cinchonine, which is applied in the direction of anti-tumor drugs, drug combinations, and resistance to vector-borne diseases, can solve the problem that cinchonine has not yet inhibited tumor cell growth, and achieves inhibition of tumor cell growth, small side effects, and toxic side effects small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1. Effect of cinchonine on tumor cell growth:
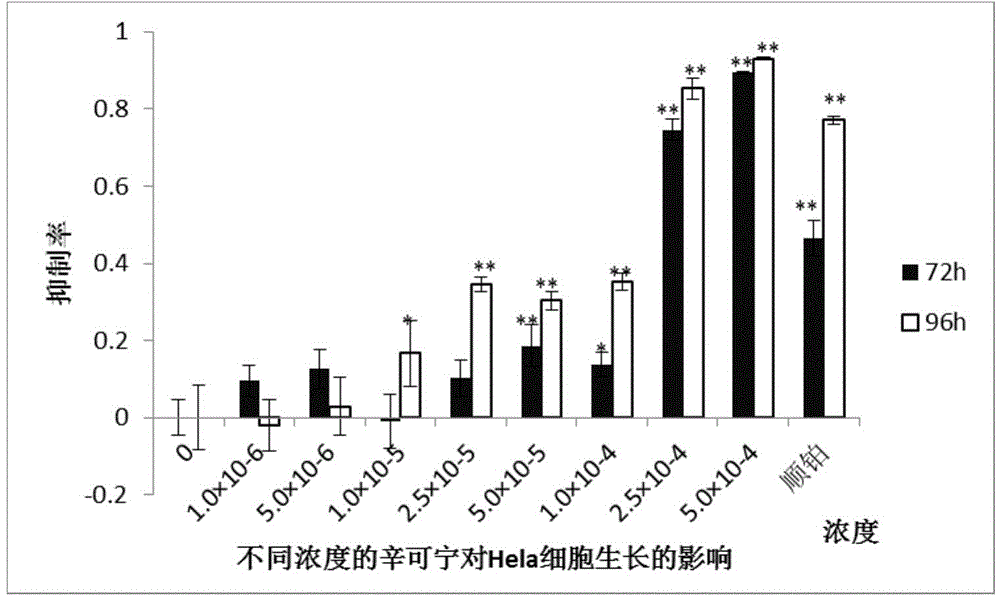
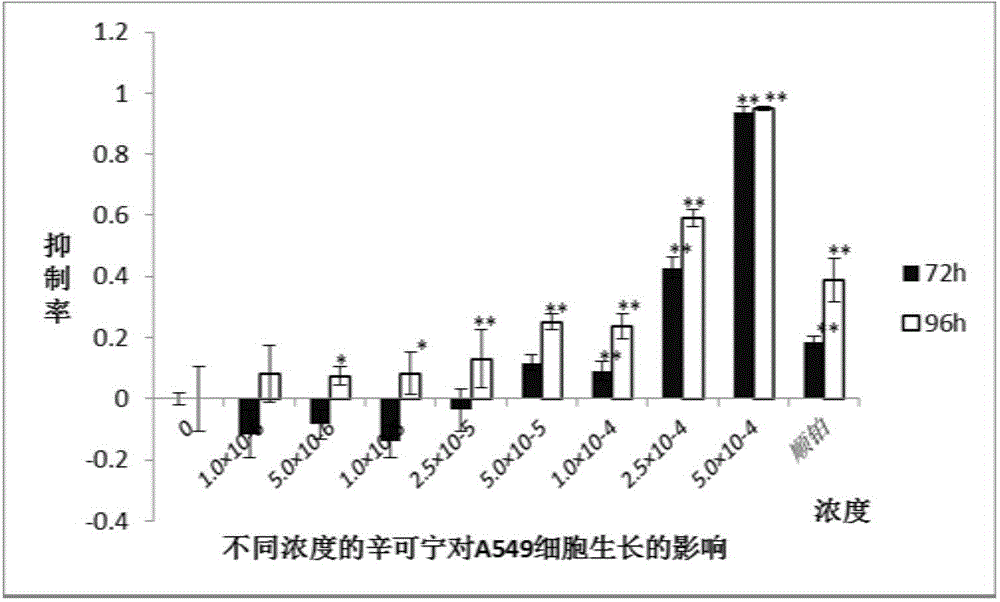
[0017] Hela cells and A-549 cells (purchased from Shenyang Wanlei Biotechnology Co., Ltd.) were cultured, seeded into 96-well plates at a cell density of 4000 cells / well, and after culturing for 24 hours (h), 1×10 -6 , 5×10 -6 , 1×10 -5 , 2.5×10 -5 , 5×10 -5 , 1×10 -4 , 2.5×10 -4 , 5×10 -4 The cinchonine of mol / L concentration, measure cell growth state with MTT method after 72h and 96h respectively, its result sees figure 1 , showing a concentration-dependent inhibition of the growth of Hela cells, figure 2 It was shown that cinchonine inhibited the growth of A-549 cells in a concentration-dependent manner.
[0018] MTT method: Add 20 μl of 5 mg / ml MTT solution to each well, take it out after incubating for 3-4 hours in the incubator, discard the supernatant, add 150 μl dimethyl sulfoxide to each well to dissolve the formazan precipitate, and use a microplate reader at 490 nm Measure the absorbance value below. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com