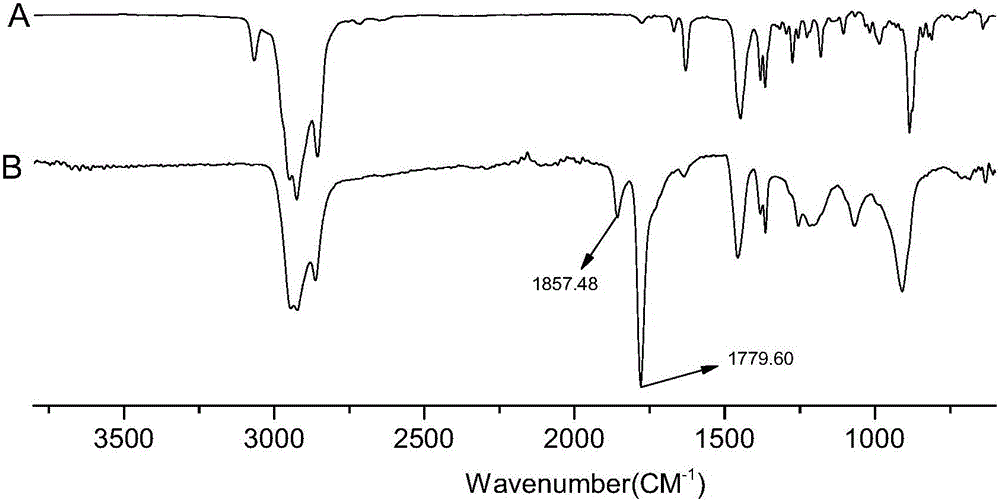
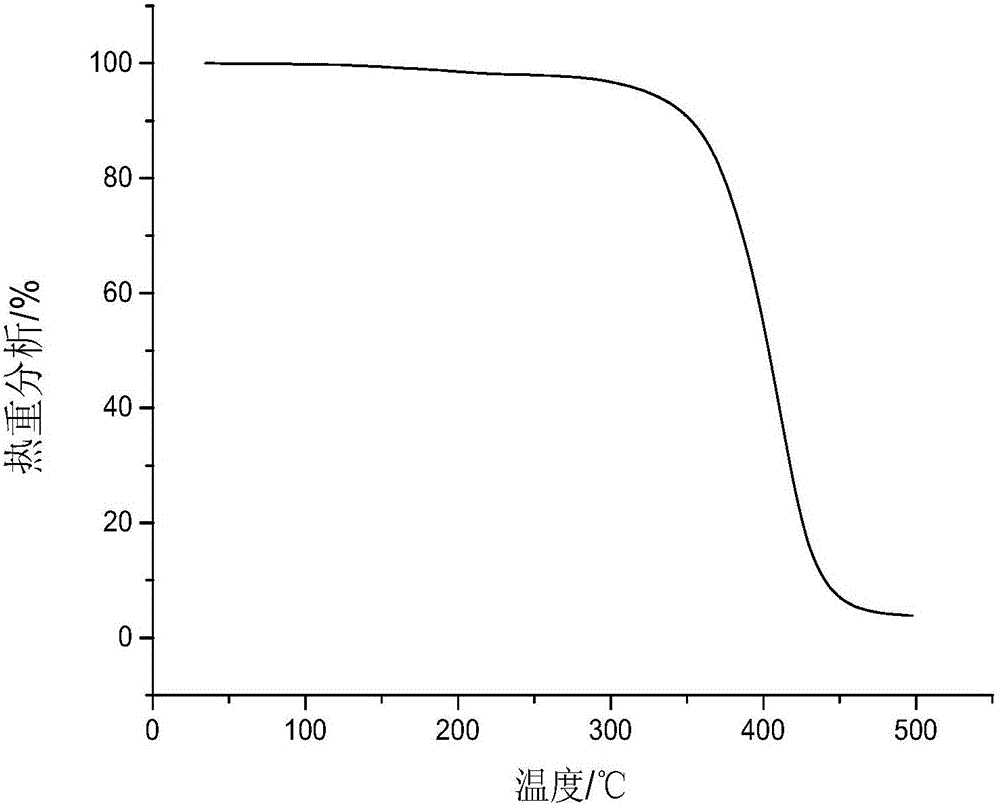
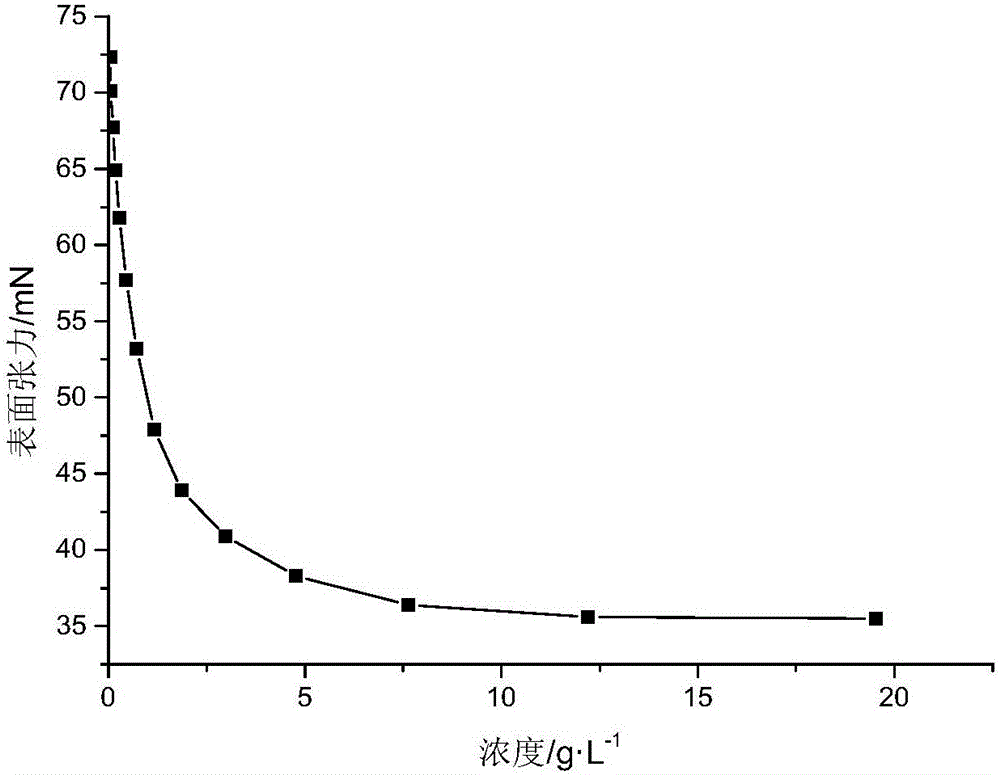
Beta-caryophyllene-maleic anhydride copolymer as well as preparation method and application thereof
A kind of technology of maleic anhydride and caryophyllene, applied in the field of beta-caryophyllene-maleic anhydride copolymer and preparation thereof, can solve the problems that do not involve beta-caryophyllene-maleic anhydride and the like, achieve mild conditions and operating costs Low, simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 10.38 g of β-caryophyllene, 4.90 g of maleic anhydride, 0.584 g of di-tert-butyl peroxide and 30.6 g of cyclohexanone into a 100 ml three-necked flask equipped with a thermometer and a magnetic stirring bar. Under the protection of nitrogen, raise the temperature to 150°C, react for 1 hour, then stop the reaction, pour the reactant into a large amount of methanol, a white precipitate is precipitated, and then perform suction filtration to obtain a white powdery solid. After drying, the measured mass is 11.1g , yield 72.63%. The molecular weight of the copolymer measured by GPC M n for 1404, M w for 3249. Then get 2g copolymer and join in the one-necked flask that the NaOH solution of 5% mass fraction is housed, heat and reflux to transparent, then pour the reactant into a large amount of methanol to separate out a white precipitate, suction filter, and dry to obtain a saponified product. The surface tension of the object was measured, and the results are shown in...
Embodiment 2
[0036] 10.38 g of β-caryophyllene, 4.90 g of maleic anhydride, 0.438 g of di-tert-butyl peroxide and 22.93 g of cyclohexanone were added. Under the protection of nitrogen, the temperature was raised to 145° C., and the reaction was carried out for 1 hour. The other conditions were the same as in Example 1. The quality of the finally obtained copolymer was 11.1 g, and the yield was 79.83%.
Embodiment 3
[0038] 10.38 g of β-caryophyllene, 4.90 g of maleic anhydride, 0.584 g of di-tert-butyl peroxide and 30.57 g of cyclohexanone were added. Under the protection of nitrogen, the temperature was raised to 150° C., and the reaction was carried out for 10 minutes. The other conditions were the same as in Example 1. The quality of the finally obtained copolymer was 9.6 g, and the yield was 62.81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com