Optical-interference-free Raman labeling probe and preparation method and application thereof
A technology for labeling probes and Raman, which is used in Raman scattering, material analysis by optical means, scientific instruments, etc. It can solve the problems of weak signal intensity of spontaneous Raman scattering, inability to become a general-purpose instrument for biological imaging, and sample damage. , to achieve the effect of shortening biological imaging time, avoiding overlapping, and eliminating interference peaks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of narrow-band single-peak Raman signal molecule
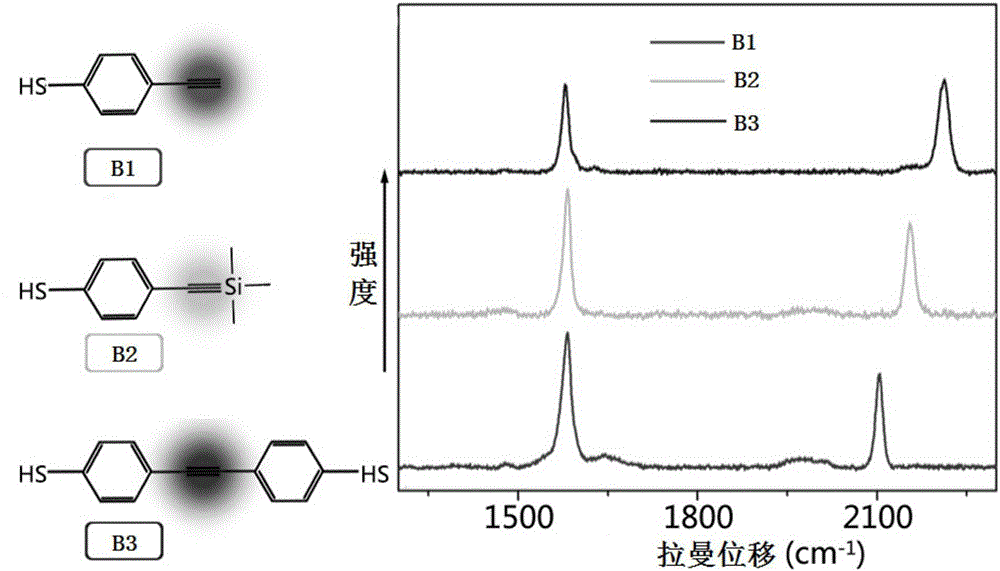
[0045] Alkyne narrow-band single-peak Raman signal molecular design: the present invention uses p-mercaptophenylacetylene as the main structure, and changes the displacement of the alkynyl Raman scattering signal by replacing the alkynyl terminal substituent group, and uses density functional theory to calculate the A series of predictions and theoretical studies have been carried out on the relationship between the structure of the signal molecule and the Raman shift, and the chemical structure and its Raman shift in the table below have been obtained, which proves that when the group is changed at the end of the alkyne group of p-mercaptophenylacetylene, it can effectively Modulation of Raman Signal Shifts of Narrowband Singlet Signaling Molecules.
[0046]
[0047] Spectrum optimization of signal molecules: Using silver-coated gold nanoparticles of a certain size as the enhanced substrate, t...
Embodiment 2
[0050] (1) AuNPs-labeled molecules: Take three 10 mL silver-coated gold nanosols, and add 10 μL of 1 mM narrow-band unimodal signal molecules B1, B2, and B3 in tetrahydrofuran respectively.
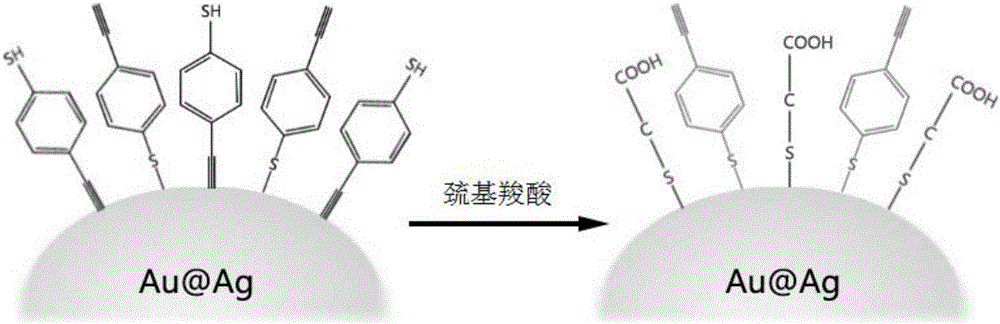
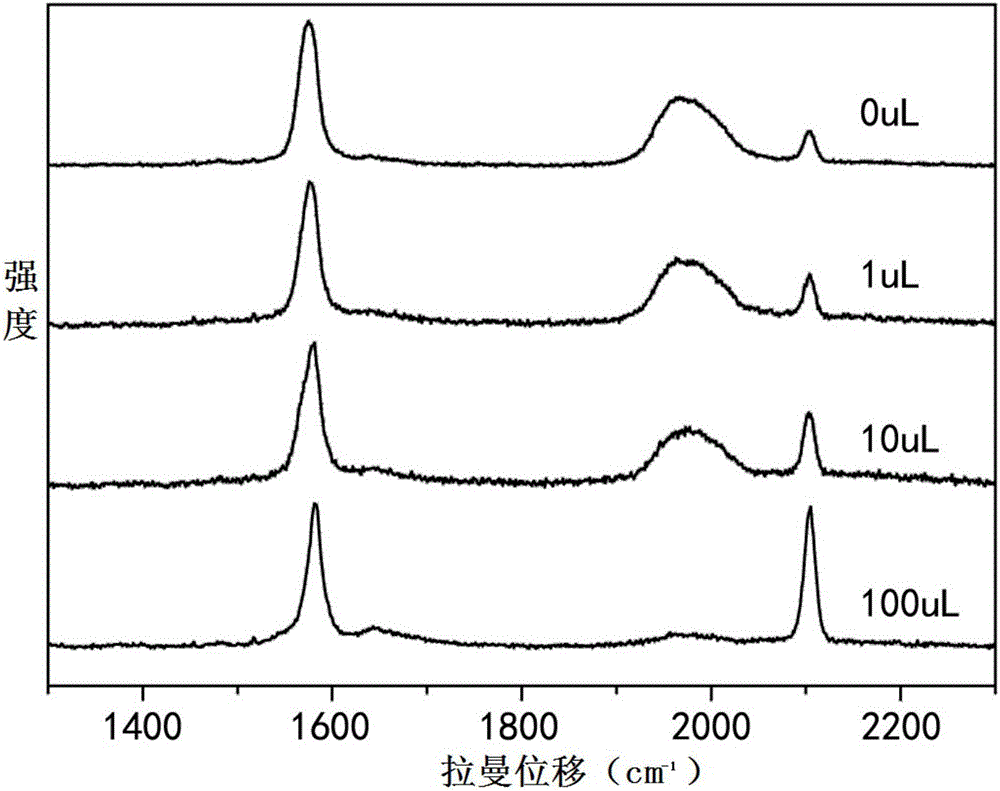
[0051] (2) Optimization of thioglycolic acid: After the above solution was allowed to stand for 3 hours, 100 μL of an aqueous solution of thioglycolic acid with a concentration of 10 mmol / L was added. (This step is only required when the alkynyl terminal of the signal molecule is not substituted, for example, B1 is used as the signal molecule).
[0052] (3) Protective shell layer: After standing still for 3 hours, continue to add 1 mL of polycyclic aromatic hydrocarbon (PAH) aqueous solution with a concentration of 0.1 wt‰.
[0053] (4) Biological functional molecule connection: 10 μL of 10 mM 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) aqueous solution and 20 μL of 1mM N-hydroxysuccinimide (NHS) aqueous solution, let stand to activate the carboxyl group, add 20...
Embodiment 3
[0057] Take 10mL particle concentration is about 10 12 silver-coated gold nano-sol per mL, add 20 μL of B3 signal molecules with a concentration of 1mM to it, and then continue to add 1mL of polycyclic aromatic hydrocarbon (PAH) aqueous solution with a concentration of 0.1wt‰ after standing for 12 hours. SERS probe.
[0058] Take 10 mL of an aqueous solution in which lily pollen is evenly dispersed, and add 1 mL of SERS probe to it, mix well on a shaker, and freeze-dry the pollen solution to remove water.
[0059] Prepare a high-concentration sucrose solution and drop a small amount onto the glass slide. After drying, a uniform and transparent sucrose layer is formed on the surface. Take a small amount of pollen grains and sprinkle them on the sucrose layer and gently blow air to apply pressure, so that the pollen can be firmly adhered to the surface of the sucrose, and the Raman imaging experiment can be performed.
[0060] Based on the flavonoids in pollen at 1580cm -1 Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com