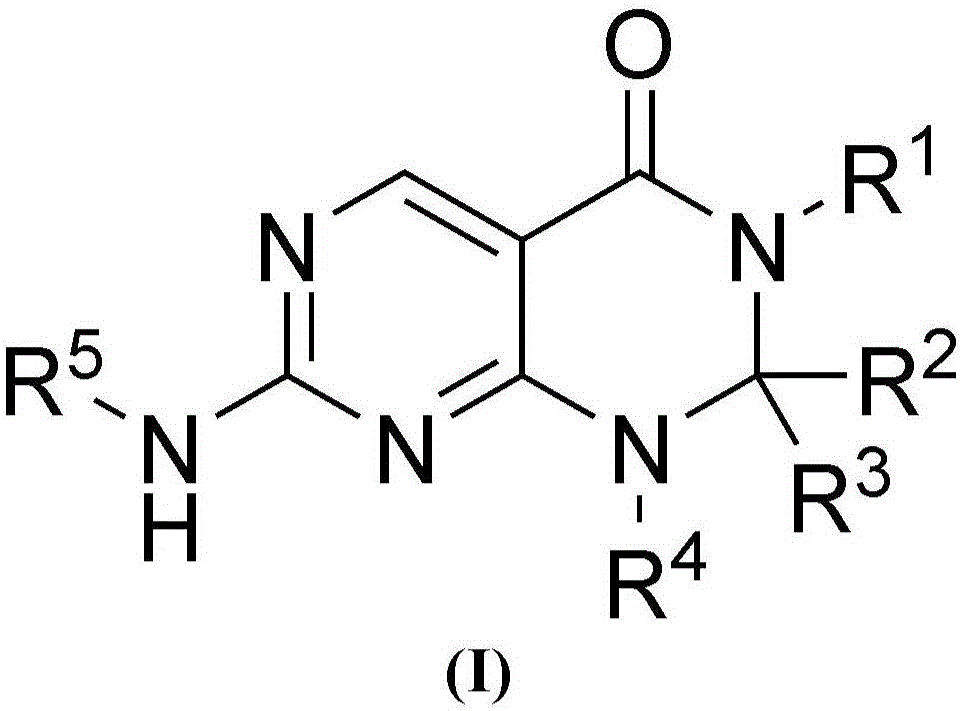
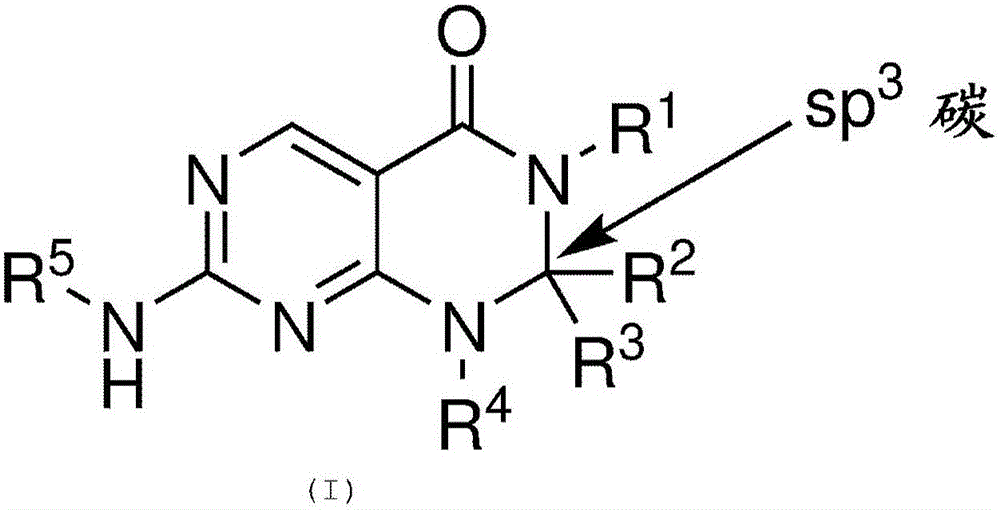
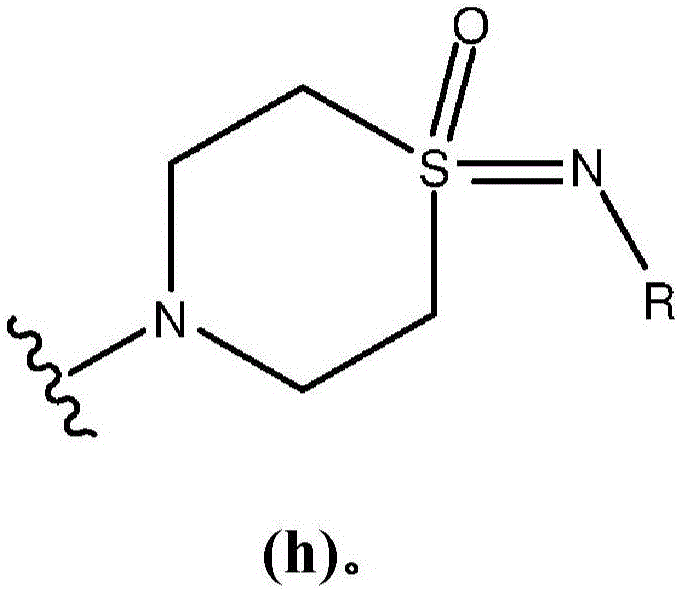
Pyrimidopyrimidinones useful as Wee-1 kinase inhibitors
A kind of medicinal salt, C1-C6 technology, applied in the compound field of Wee-1 kinase activity inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0303] Example 1: 3-(2,6-dichlorophenyl)-1-methyl-7-((4-(piperazin-1-yl)phenyl)amino)-2,3-dihydro pyrimido[4,5-d]pyrimidin-4(1H)-one
[0304]
[0305] Step 1: N-(2,6-Dichlorophenyl)-4-(methylamino)-2-(methylthio)pyrimidine-5-carboxamide:
[0306]4-(methylamino)-2-(methylthio)pyrimidine-5-carboxylic acid [Bioorg.Med.Chem.2005,13(16),4936](0.5g, 2.51mmol) was suspended in chlorobenzene ( 10 mL) and added 2,6-dichloroaniline (0.407 g, 2.51 mmol). Phosphorus trichloride (0.220 mL, 2.51 mmol) was added and the mixture was stirred at 120°C for 16 hours. React with 2M Na 2 CO 3 The aqueous solution was quenched, then extracted with ethyl acetate (x2). The combined organic extracts were washed with brine and dried (MgSO 4 ), filtered and concentrated (azeotrope with toluene). The residue was triturated with diethyl ether and the title compound was collected by filtration to give the title compound (180 mg, 21%) as a yellowish solid. The mother liquor was concentrated to ...
Embodiment 2
[0317] Example 2: 3-(2,6-dichlorophenyl)-1-methyl-7-((4-(2-(methylamino)ethoxy)phenyl)amino)-2, 3-Dihydropyrimido[4,5-d]pyrimidin-4(1H)-one
[0318]
[0319] According to the method of Example 1, 3-(2,6-dichlorophenyl)-1-methyl-7-(methylthio)-2,3-dihydropyrimido[4,5-d]pyrimidine -4(1H)-Kone (82 mg, 0.231 mmol) was reacted with (2-(4-aminophenoxy)ethyl)(methyl)carbamate tert-butyl ester (61.5 mg, 0.231 mmol) to give The title compound (27mg, 24%).
[0320] 1 HNMR (500MHz, DMSO-d 6 ): δ9.71(s, 1H), 8.45(s, 1H), 7.69(m, 4H), 7.47(m, 1H), 6.89(d, 2H), 4.88(s, 2H), 3.97(m, 2H), 3.10(s, 3H), 2.81(t, 2H), 2.32(s, 3H).
[0321] LCMS (Method A): R T = 0.77 minutes, m / z = 473 [M+H] + .
Embodiment 3
[0322] Example 3: 3-(2,6-dichlorophenyl)-7-((3-methoxy-4-(piperazin-1-yl)phenyl)amino)-1-methyl - 2,3-Dihydropyrimido[4,5-d]pyrimidin-4(1H)-one
[0323]
[0324] According to the method of Example 1, 3-(2,6-dichlorophenyl)-1-methyl-7-(methylthio)-2,3-dihydropyrimido[4,5-d]pyrimidine - 4(1H)-one (50 mg, 0.141 mmol) was reacted with tert-butyl 4-(4-amino-2-methoxyphenyl)piperazine-1-carboxylate (43 mg, 0.14 mmol) to give The title compound as a solid (34 mg, 47%).
[0325] 1 HNMR (500MHz, DMSO-d 6 ): δ9.75(s, 1H), 8.48(s, 1H), 7.65(m, 3H), 7.47(m, 1H), 7.21(d, 1H), 6.85(d, 1H), 4.97(s, 2H), 3.69(s, 3H), 3.13(s, 3H), 2.92(m, 8H).
[0326] LCMS (Method A): R T = 0.75 minutes, m / z = 514 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com