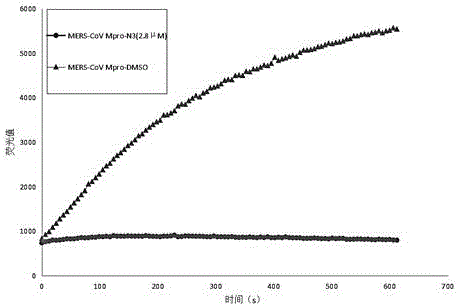
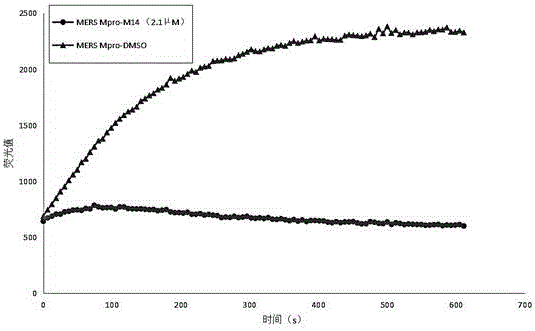
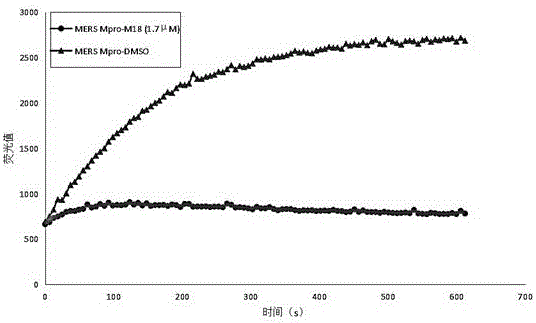
Small-molecule inhibitor against MERS-CoV main protease, and preparation method and application thereof
A technology of small molecule inhibitors and proteases, applied in the fields of antiviral agents, bulk chemical production, organic chemistry, etc., can solve the problems of long clinical trials, poor specificity, no specific drugs, etc., and achieve good application prospects, good The effect of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0085] The preparation method of MERS-CoV main protease small molecule inhibitor of the present invention comprises the following steps:
[0086] A, the amino protecting group R in the corresponding compound of general formula (II) 6 removed, where the R 6 Selected from the group consisting of the following groups: tert-butoxycarbonyl, trifluoroacetyl, benzyloxycarbonyl, methoxycarbonyl, allyloxycarbonyl;
[0087] B, in the presence of a condensing agent, the product of step A is condensed with the corresponding compound of general formula (III) to obtain general formula (I),
[0088]
[0089] Wherein, R in general formula (II) and general formula (III) 1 , R 2 , R 3 , the definitions of U are the same as R in the general formula (I) 1 , R 2 , R 3 , U have the same definition.
[0090] In the preparation method of the small molecule inhibitor of MERS-CoV main protease, the following steps are also included: in an organic solvent, the compound corresponding to the ge...
Embodiment 1
[0103] 300mg of Dissolve in 3mL
[0104] Dry CH 2 Cl 2 , add 0.49g sodium bicarbonate, then add 1.23g Dess Martin reagent, react at room temperature for 8-12 hours, quench the reaction with sodium thiosulfate solution, wash with saturated saline and spin dry to obtain Dissolve with dry THF5ml and set aside. 0.52g Dissolve in 5ml of dry THF, cool and stir at -78°C, then add 60% NaH 0.078g and stir for 1h, then slowly add the ready-to-use Solution, turn off the refrigeration, react for 12h, quench the reaction with saturated ammonium chloride, spin dry the THF in the system, and extract the product with EA. Flash silica gel column chromatography, obtain 82mg product Dissolve the resulting product in 1 mL CH 2 Cl 2 , add 58 μL TFA, stir at room temperature for 2 hours, drain the solvent to obtain the product deprotected by Boc, and then dissolve it in 5ml of CH 2 Cl 2 , add 158mg
[0105] and 257uL DIEA, followed by 220mg HATU. The system was reacted at room te...
Embodiment 2
[0111] 200mg Dissolve in 3ml CH 2 Cl 2 In, add 220μl DIEA and 140mg Stir at room temperature for 12h, and obtain 100mg by silica gel column chromatography
[0112] Dissolve the resulting product in 1 mL CH 2 Cl 2 , add 58 μL TFA, stir at room temperature for 2 hours, drain the solvent to obtain the product deprotected by Boc, and then dissolve it in 3ml of CH 2 Cl 2 , add 192mg
[0113] And 320ul DIEA, followed by 380mg HATU. The system was reacted at room temperature for 12 hours, followed by 1M HCl, saturated NaHCO 3 aqueous solution, saturated brine, and then collected the organic phase, anhydrous Na 2 SO 4 dry, the Na 2 SO 4 Filtered out, the organic phase was evaporated under reduced pressure to remove the solvent, followed by flash silica gel column chromatography to obtain 80 mg of product M18
[0114] Product hydrogen spectrum and mass spectrum data are as follows:
[0115] 1 H NMR (400MHz, DMSO-d6): δ8.59 (d, J=7.5Hz, 1H, NH), 8.18-8.28 (m, 1H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com