Indole derivatives containing piperazinyl group and its preparation method and application
A technology of indole and piperazine, applied in the field of indole derivatives containing piperazine group and its preparation and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
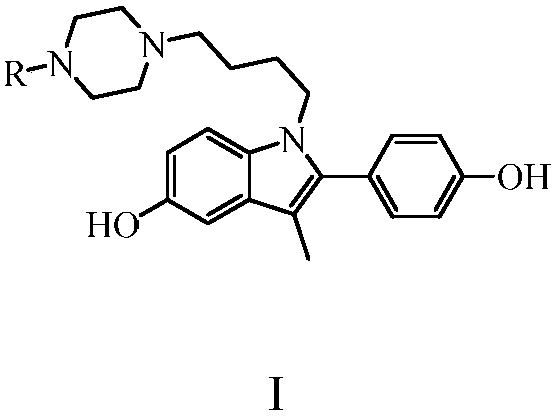
[0033] Example 1: Preparation of 1-[4-(4-methylpiperazin-1-yl)butyl]-5-hydroxyl-2-(4-hydroxyphenyl)-3-methyl-1H-indole
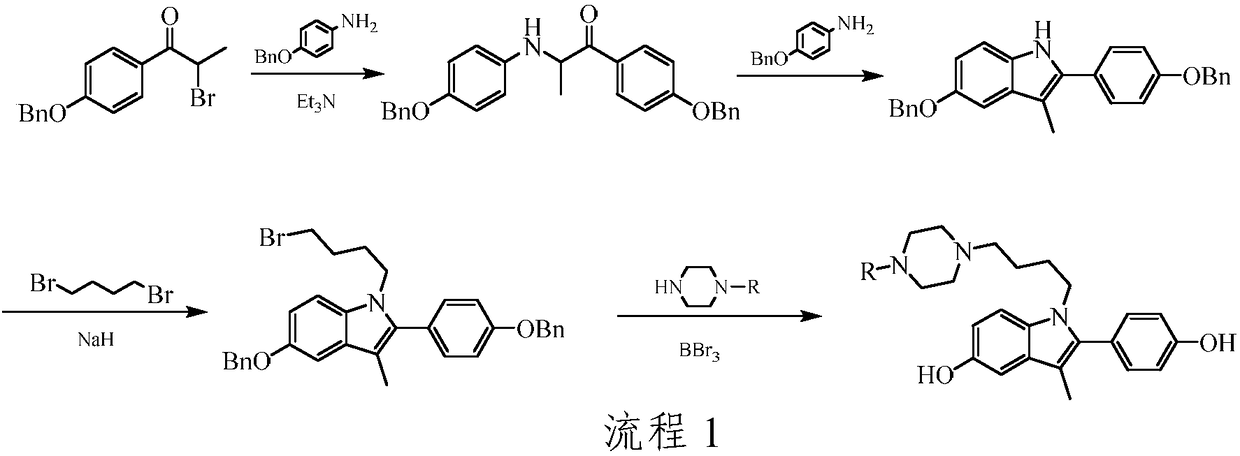
[0034] Step A): Preparation of 1-(4-benzyloxyphenyl)-2-(4-benzyloxyphenylamino)-1-propanone
[0035] Place 4-benzyloxyaniline (7.8g, 0.039mol), 1-(4-benzyloxyphenyl)-2-bromo-1-propanone (10.5g, 0.033mol) in a 500mL round bottom flask, ethanol As a solvent, triethylamine (6.6g, 0.065mol) was added, and the reaction was refluxed for 5h. Naturally cooled to room temperature, and suction filtered to obtain 12.3 g of a milky white solid with a yield of 85.3%. m.p.125-126°C. ESI-MS: m / z 438 ([M+H] + ).
[0036] Step B): Preparation of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole
[0037] 1-(4-benzyloxyphenyl)-2-(4-benzyloxyphenylamino)-1-propanone (12.3g, 0.028mol), 4-benzyloxyaniline (14.0g, 0.070mol) Place in a 500mL flask, use ethylene glycol monoethyl ether as a solvent, and reflux for 6 hours. The solvent was evaporated under reduced pressure, ...
Embodiment 2
[0042] Example 2: Preparation of 1-[4-(4-ethylpiperazin-1-yl)butyl]-5-hydroxyl-2-(4-hydroxyphenyl)-3-methyl-1H-indole
[0043] According to the method of Example 1, 0.31 g of a yellow solid was obtained with a yield of 36.7%. m.p.106-107°C. ESI-MS: m / z 408 ([M+H] + ). 1 H-NMR (400MHz, DMSO-d 6 )δ8.53(s,1H),7.96(s,1H),7.23(d,J=8.7Hz,1H),7.17(d,J=8.6Hz,2H),6.89(d,J=8.5Hz, 2H), 6.77(d, J=2.2Hz, 1H), 6.64(dd, J=8.4, 1.9Hz, 1H), 4.22(t, J=6.6Hz, 2H), 3.95(t, J=7.1Hz, 2H),3.62(m,2H),3.47(m,2H),2.98(m,2H),2.67(m,2H),2.18(m,2H),2.04(s,3H),1.62(m,4H ), 1.06 (t, J=5.6Hz, 3H).
Embodiment 3
[0044] Example 3: Preparation of 1-[4-(piperazin-1-yl)butyl]-5-hydroxyl-2-(4-hydroxyphenyl)-3-methyl-1H-indole
[0045] According to the method of Example 1, 0.32 g of an orange solid was obtained, with a yield of 41.9%. m.p.155-156°C. ESI-MS: m / z380 ([M+H] + ). 1 H-NMR (400MHz, DMSO-d 6 )δ9.78(s,1H),8.52(s,1H),7.23(d,J=8.7Hz,1H),7.17(d,J=8.6Hz,2H),6.89(d,J=8.5Hz, 2H), 6.77(d, J=2.2Hz, 1H), 6.64(dd, J 1 =8.7Hz,J 2 =2.2Hz,1H),4.22(t,J=6.6Hz,2H),3.95(m,2H),3.64(m,2H),3.47(m,2H),2.98(m,2H),2.39(s , 1H), 2.18(t, J=7.1Hz, 2H), 2.04(s, 3H), 1.07(m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com