Asymmetrical iridium (III) phosphorescent complex containing dibenzo-phosphorus mixed with cyclopentadienyl group, and synthesis method of asymmetrical iridium (III) phosphorescent complex
A phosphorescent complex, benzophosphine technology, which is applied in the field of asymmetric iridium phosphorescent complexes and their synthesis, can solve the problems such as the development lag of phosphorescent materials, and achieves improved electroluminescence efficiency, good carrier balance, and fully converted technology. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
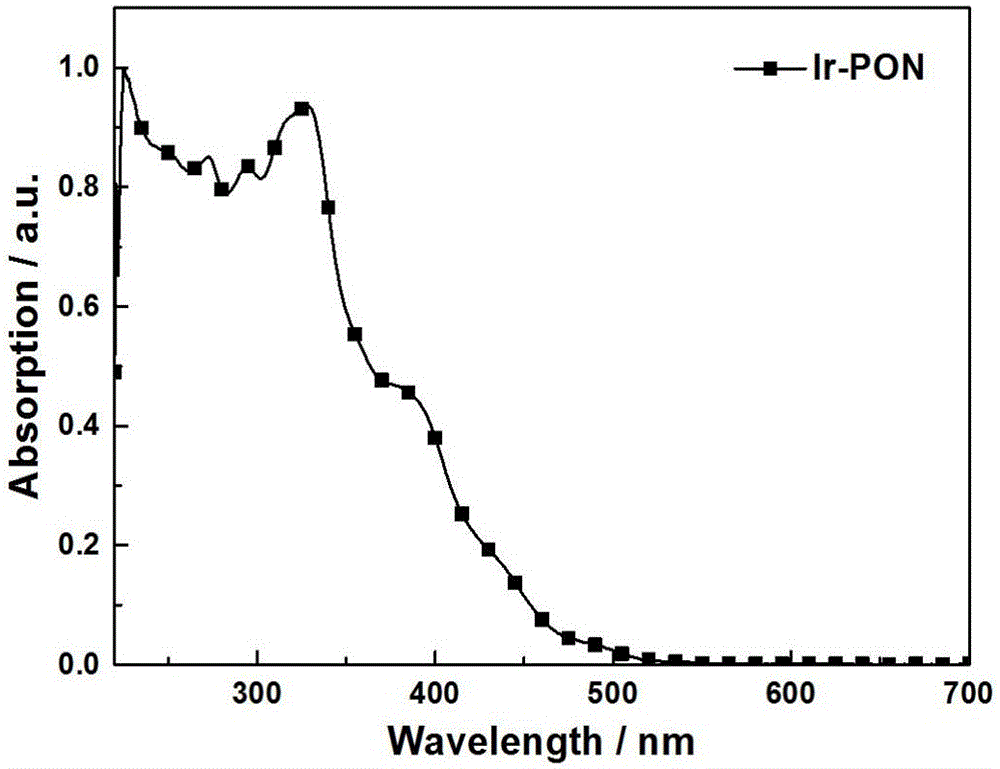
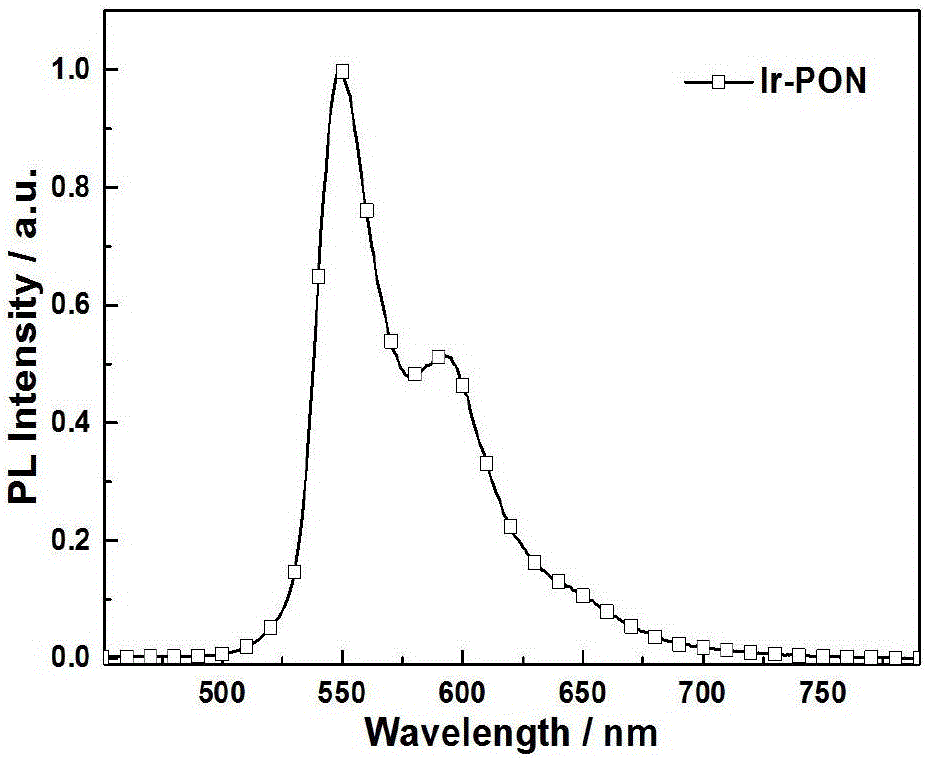
[0037] Asymmetric iridium (III) phosphorescent complex Ir-PON containing dibenzophosphine group, its chemical formula is: C 51 h 39 IrN 3 o 3 P, the molecular structural formula is:
[0038]
[0039] Its synthetic method comprises the following steps:
[0040] Step 1: Obtain the ligand L-PO according to the synthesis method of the above-mentioned ligand containing diphenylphosphine group;
[0041] Step 2: Under a nitrogen atmosphere, weigh 0.35g (1mmol) of ligand L-PO, 0.32g (1mmol) of ligand L-N (for the synthesis of ligands, see references Adv.Funct.Mater.2008,18,499–511) and Add 0.32g of iridium trichloride hydrate (60% iridium content) into the SCHLENK reaction tube, add 30mL of degassed mixed solvent of ethylene glycol ether and water (V:V=3:1), heat up to 110°C and stir for reaction 16 Hour. After the reaction system was cooled to room temperature, an appropriate amount of brine was added to the reaction mixture, and a precipitate appeared. Take the filter cake...
Embodiment 2
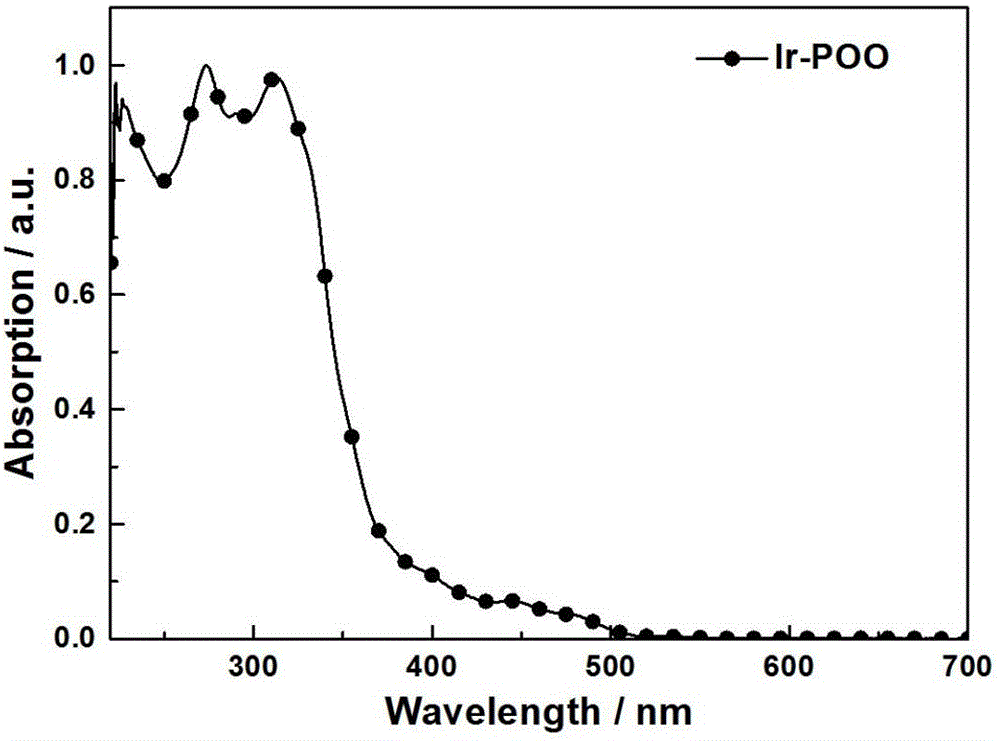
[0045] Asymmetric iridium(III) phosphorescent complex Ir-POO containing dibenzophosphine group, the formula is: C 45 h 34 IrN 2 o 4 P, the molecular structural formula is:
[0046]
[0047] Its synthetic method comprises the following steps:
[0048] Step 1: Obtain the ligand L-PO according to the synthesis method of the above-mentioned ligand containing diphenylphosphine group;
[0049] Step 2: Under a nitrogen atmosphere, weigh 0.35g (1mmol) of ligand L-PO, 0.25g (1mmol) of ligand L-O (for the synthesis of ligands, see references Adv.Funct.Mater.2008,18,499–511) and Add 0.32g of iridium trichloride hydrate (iridium content 60%) into the SCHLENK reaction tube, add 30mL of degassed mixed solvent of ethylene glycol ether and water (V:V=3:1), heat up to 110°C and stir for 16 hours . After the reaction system was cooled to room temperature, an appropriate amount of brine was added to the reaction mixture, and a precipitate appeared. Take the filter cake by suction filtr...
Embodiment 3
[0053] Asymmetric iridium (III) phosphorescent complex Ir-POB containing dibenzophosphine group, its chemical formula is: C 57 h 51 BYZGR 2 o 3 P, the molecular structural formula is:
[0054]
[0055] Its synthetic method comprises the following steps:
[0056] Step 1: Obtain the ligand L-PO according to the synthesis method of the above-mentioned ligand containing diphenylphosphine group;
[0057] Step 2: Under a nitrogen atmosphere, weigh 0.35g (1mmol) of ligand L-PO, 0.41g (1mmol) of ligand L-B (for the synthesis of ligands, see references Adv.Funct.Mater.2008,18,499–511) and Add 0.32g of iridium trichloride hydrate (60% iridium content) into the SCHLENK reaction tube, add 30mL of degassed mixed solvent of ethylene glycol ether and water (V:V=3:1), heat up to 110°C and stir for reaction 16 Hour. After the reaction system was cooled to room temperature, an appropriate amount of brine was added to the reaction mixture, and a precipitate appeared. Take the filter ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com