Spirostanol saponins and application thereof
A compound and spirosteroid technology, which is applied to spirosteroids and their application fields, can solve the problem of insufficient research on chemical components of open arrows.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Extraction and identification of spiro steroids
[0028] 1. Extraction of spiro steroids
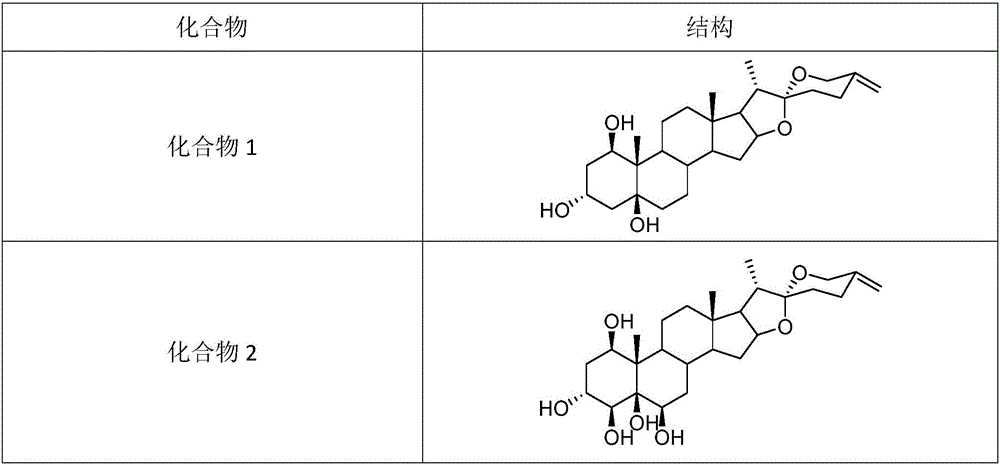
[0029] Take 17 kg of open arrow rhizomes, heat and reflux with 60% ethanol to extract 4 times, each time for 4 hours, combine the extracts to recover the solvent under reduced pressure, and obtain the total extract, suspend the total extract with water, and extract with equal volume of ethyl acetate for 4 Next, fat-soluble impurities were removed, and the aqueous layer was separated by D101 macroporous resin column chromatography, and eluted with water, 20% ethanol, 60% ethanol, 80% ethanol, and 95% ethanol respectively to obtain 5 fractions TA, TB, TC, TD, TE. Take fraction TC (60% ethanol eluted part) 200g, adopt silica gel column chromatography, ODS medium and low pressure column chromatography, reverse phase high performance liquid chromatography and other separation methods to separate and obtain compounds 1 and 2 of the present invention. Compound 3-9 of the pres...
Embodiment 2
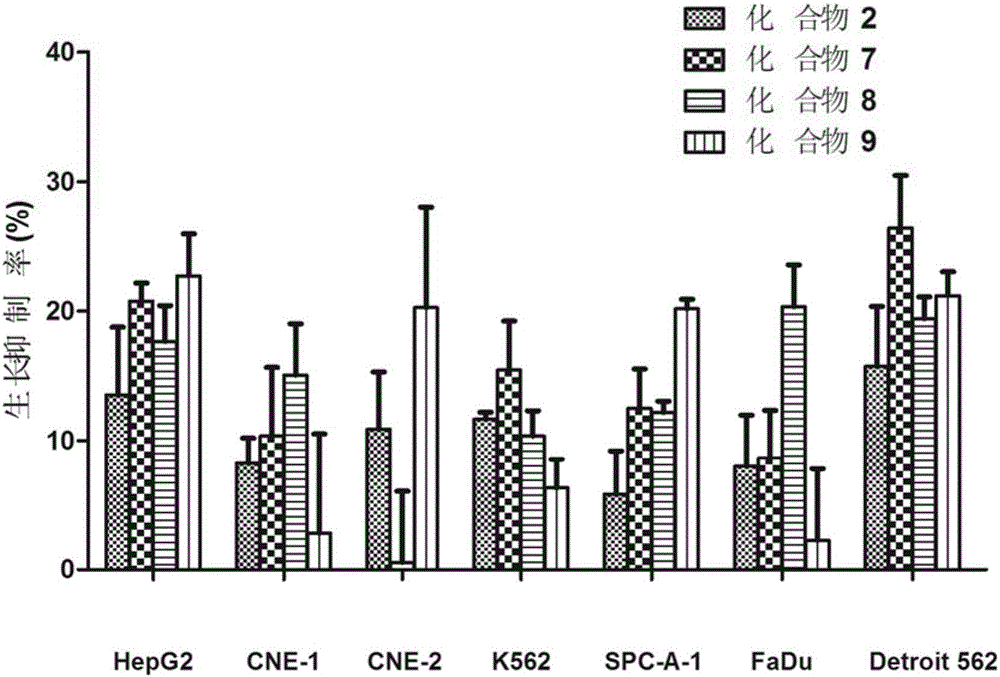
[0086] Embodiment 2 Tumor inhibition test:
[0087] The compound of the present invention is to the in vitro antitumor activity experiment of 7 tumor strains of human body, these 7 tumor strains comprise FaDu (human pharyngeal squamous cell carcinoma cell), Detroit 562 (human pharyngeal carcinoma pleural effusion metastasis cell), CNE-1 (well-differentiated human Nasopharyngeal squamous cell carcinoma cells), CNE-2 (poorly differentiated human nasopharyngeal squamous cell carcinoma cells), HepG2 (human liver cancer cells), K562 (human chronic myelogenous leukemia cells), SPC-A-1 (human lung adenocarcinoma cells) Cytotoxic activity of seven human tumor cells and toxicity to normal cell L-929 (mouse fibroblasts). The anticancer drug cis-dichlorodiamineplatinum (II) (cisplatinum) was used as a positive control.
[0088] Inhibition of tumor cell proliferation (MTT method)
[0089] The tumor cells were inoculated in a 96-well plate, and after 24 hours of culture, the samples to b...
Embodiment 3
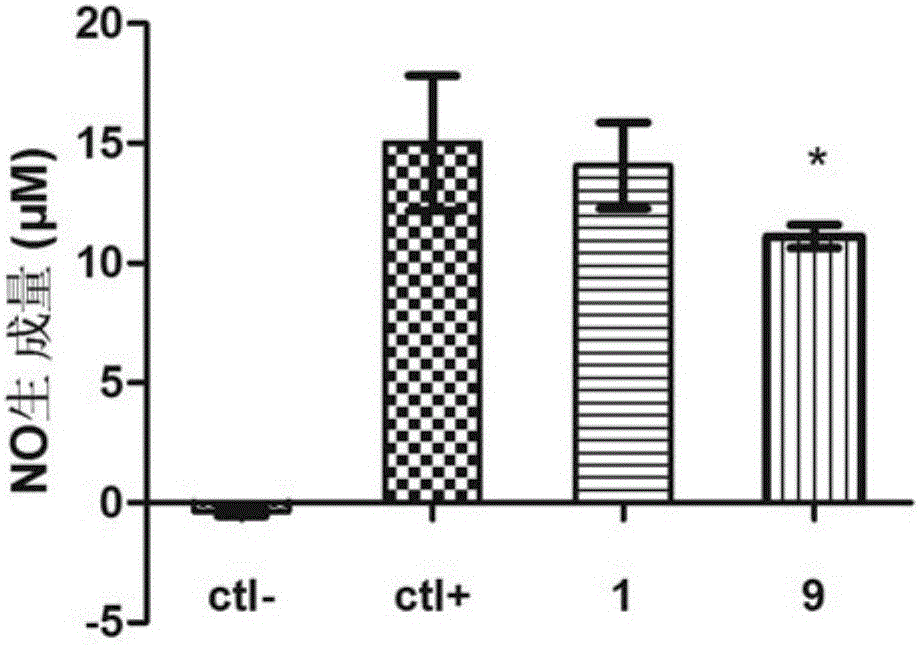
[0095] Embodiment 3 anti-inflammatory test:
[0096] For the anti-inflammatory activity test of the compound of the present invention in vitro, Raw 264.7 (mouse macrophage) cells induced by LPS (lipopolysaccharide) were used to establish an in vitro inflammation model. MTT and Griess experiments were used to investigate the effect of the compound of the present invention on the release of inflammatory mediator NO from Raw 264.7 cells induced by lipopolysaccharide. The anti-inflammatory drug Indomethacin (indomethacin) was used as a positive control.
[0097] 1) MTT experiment
[0098] Raw 264.7 cells were inoculated in a 96-well plate, and after 24 hours of culture, the product to be tested was added, and after another 24 hours of culture, the inhibition rate of the sample on tumor cell proliferation was measured by the MTT method. The cell proliferation inhibition rate was calculated according to the following formula, and the half inhibitory concentration (IC) of the teste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com